- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Current Chemical Genomics and Translational Medicine
(Discontinued)
ISSN: 2213-9885 ― Volume 12, 2018
Hepatocellular Carcinoma: Causes, Mechanism of Progression and Biomarkers
Amit Kumar Singh, Ramesh Kumar, Abhay K. Pandey*
Abstract
Hepatocellular Carcinoma (HCC) is one of the most common malignant tumours in the world. It is a heterogeneous group of a tumour that vary in risk factor and genetic and epigenetic alteration event. Mortality due to HCC in last fifteen years has increased. Multiple factors including viruses, chemicals, and inborn and acquired metabolic diseases are responsible for its development. HCC is closely associated with hepatitis B virus, and at least in some regions of the world with hepatitis C virus. Liver injury caused by viral factor affects many cellular processes such as cell signalling, apoptosis, transcription, DNA repair which in turn induce important effects on cell survival, growth, transformation and maintenance. Molecular mechanisms of hepatocellular carcinogenesis may vary depending on different factors and this is probably why a large set of mechanisms have been associated with these tumours. Various biomarkers including α-fetoprotein, des-γ-carboxyprothrombin, glypican-3, golgi protein-73, squamous cell carcinoma antigen, circulating miRNAs and altered DNA methylation pattern have shown diagnostic significance. This review article covers up key molecular pathway alterations, biomarkers for diagnosis of HCC, anti-HCC drugs and relevance of key molecule/pathway/receptor as a drug target.
Article Information
Identifiers and Pagination:
Year: 2018Volume: 12
First Page: 9
Last Page: 26
Publisher Id: CCGTM-12-9
DOI: 10.2174/2213988501812010009
Article History:
Received Date: 11/04/2018Revision Received Date: 15/05/2018
Acceptance Date: 20/05/2018
Electronic publication date: 29/06/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Biochemistry, University of Allahabad, Allahabad 211002, India; Tel: +91 98395 21138; E-mail: akpandey23@rediffmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 11-04-2018 |
Original Manuscript | Hepatocellular Carcinoma: Causes, Mechanism of Progression and Biomarkers | |
1. INTRODUCTION
Liver cancer is one of the leading causes of cancer deaths worldwide. In recent years, the annual death toll with 700,000 has been recorded around the globe [1Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5): E359-86.
[http://dx.doi.org/10.1002/ijc.29210] [PMID: 25220842] ]. Hepatocellular Carcinoma (HCC) is the major form of liver cancer. Risk factors for HCC include chronic HBV (hepatitis B virus) and HCV (hepatitis C virus) infections, autoimmune hepatitis, chronic alcohol use, obesity and diabetes mellitus etc [2Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol 2010; 7(8): 448-58.
[http://dx.doi.org/10.1038/nrgastro.2010.100] [PMID: 20628345] ]. Between 1990 and 2013, about 63% increase in total deaths has been reported globally because of viral hepatitis. Hepatitis B and C infections accounted for most of the morbidity and mortality since it leads to progressive hepatic damage in patients and ultimately causing cirrhosis and hepatocellular carcinoma [3Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: Findings from the global burden of disease study 2013. Lancet 2016; 388(10049): 1081-8.
[http://dx.doi.org/10.1016/S0140-6736(16)30579-7] [PMID: 27394647] ].
In areas of high incidence, HCC has been reported in children of even two years of age. However, the incidence increases with age in all populations and shows a slight decline in the elderly population. HCC shows a strong male preference. In low incidence regions, it is four times more common in males while in high prevalence areas, it is about eight times more common. This report may be partially ascribed to the collective effect of other factors including higher levels of alcohol intake and smoking coupled with a higher incidence of cirrhosis in males. Animal experiments have suggested the role of sex hormones and/or hormone receptors. Orchidectomy reduces the carcinogenic effects of chemicals in male rats to the level found in females. A similar effect has been observed with stilbesterol or estradiol pellets’ implantation but the effect was comparatively less [4Grasso P. Experimental liver tumours in animals. In: Williams R, Johnson PJ, (eds.) Liver tumours. Bailliere’s clinical gastroenterology. 1987; London: Bailliere. pp. 183–305.].
In western countries, inborn errors of metabolism and congenital abnormalities have also contributed towards HCC in some cases [5Bianchi L. Glucogen storage disease I and hepatocellular carcinoma. Eur J Pediatr 1993; 152(Sl):S63-70. [PMID: 8391447]]. The current review describes the varied causes, molecular mechanism, biomarkers and drug targets for the diagnosis and prognosis of hepatocellular carcinoma.
2. GENETIC AND CONGENITAL ABNORMALITIES
Inbred strains of mice have shown genetic susceptibility to cirrhosis and liver cancer. However, in man, it has not been documented. Chinese and Alaskan inhabitants display familial clustering of HCC [6Alberts SR, Lanier AP, McMahon BJ, et al. Clustering of hepatocellular carcinoma in Alaska Native families. Genet Epidemiol 1991; 8(2): 127-39.
[http://dx.doi.org/10.1002/gepi.1370080206] [PMID: 1655562] , 7Leong TYM, Leong ASY. Epidemiology and carcinogenesis of hepatocellular carcinoma. HPB 2005; 7(1): 5-15.
[http://dx.doi.org/10.1080/13651820410024021] [PMID: 18333156] ]. The occurrence of HCC is rarely reported in congenital hepatic fibrosis, ataxia telangiectasia, familial polyposis coli, familial cholestatic cirrhosis, fetal alcohol syndrome, situs inversus and neurofibromatosis [7Leong TYM, Leong ASY. Epidemiology and carcinogenesis of hepatocellular carcinoma. HPB 2005; 7(1): 5-15.
[http://dx.doi.org/10.1080/13651820410024021] [PMID: 18333156] ]. Hereditary tyrosinemia, an inborn error of metabolism, is associated with the maximum risk of liver carcinoma [8Weinberg AG, Mize CE, Worthen HG. The occurrence of hepatoma in the chronic form of hereditary tyrosinemia. J Pediatr 1976; 88(3): 434-8.
[http://dx.doi.org/10.1016/S0022-3476(76)80259-4] [PMID: 173827] ]. Within a short span of time, these patients exhibited faster development of macro-nodular cirrhosis from micronodular cirrhosis, followed by dysplasia and finally HCC. Adenomas may be associated with type I glycogen storage disease but the occurrence of carcinoma is rare. Carcinogenic properties have been attributed to iron through free radical production [9Kumar S, Pandey AK. Free Radicals: Health implications and their mitigation by herbals. Br J Med Med Res 2015; 7(6): 438-57.
[http://dx.doi.org/10.9734/BJMMR/2015/16284] ]. An autosomal recessive disorder, Wilson’s disease, has a tendency to affect male population usually and causes cirrhosis via copper build up in the hepatic cells. Deficiency of alpha-1-antitrypsin, a protease inhibitor, is related to jaundice and cirrhosis during infancy, as well as with pulmonary emphysema and cirrhosis in adults [10Fregonese L, Stolk J. Hereditary alpha-1-antitrypsin deficiency and its clinical consequences. Orphanet J Rare Dis 2008; 3: 16.
[http://dx.doi.org/10.1186/1750-1172-3-16] [PMID: 18565211] ].
3. HEPATITIS VIRUS
The hepatitis viruses are unrelated human pathogens and are referred to as types A, B, C, D and E. HCC is one among ten most widespread cancers globally, is strongly related with HBV, and in some regions with HCV. HBV is a small encapsulated DNA virus having unusual reverse transcriptase activity [11Ganem D, Schneider RJ. Hepadnaviridae and their replication. In: Knipe, D.M., Howley, P.M., Griffin, D.E., Martin, M.A., Lamb, R.A., Roizman, B., et al., (eds.) 2001; Fields Virology. Vol. 4. Philadelphia, PA: Lippincott-Raven Publishers., 12Hollinger FB, Liang TJ. Hepatitis B virus. In: Knipe DM, Howley PM, Griffin DE, Lamb R.A Martin MA, Roizman B, et al. (eds.), Fields Virology. Vol. 4. Philadelphia, PA: Lippincott-Raven Publishers. 2001; 2971-3036.]. It belongs to family hepadnaviridae and has eight genotypes, A to H which have separate geographic distribution. It contains four overlapping transcription units encoding the nucleocapsid or core proteins consisting of the hepatitis B core antigen (HBcAg), the envelope proteins consisting of the Hepatitis B surface Antigen (HBsAg), the polymerase and the X protein (HBx) which has transcriptional trans-activating potential. The infectious viral particle, also known as Dane particle, is a spherical, double walled structure (diameter 42 nm) having a lipid envelope with HBsAg surrounding an inner nucleocapsid consisting of hepatitis B core antigen (HBcAg) complexed with a virally encoded polymerase and the viral DNA (Fig. 1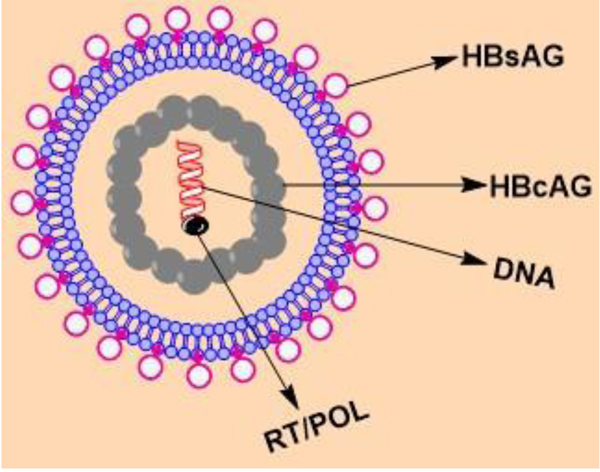 ) HBV genome is 3.2 kb in size and made up of a partially double-stranded circular DNA. The 5′ terminus of the minus strand is covalently attached to the viral polymerase.
) HBV genome is 3.2 kb in size and made up of a partially double-stranded circular DNA. The 5′ terminus of the minus strand is covalently attached to the viral polymerase.
 |
Fig. (1) Structure of HBV. |
Hepatitis C Virus (HCV) is also a member of hepadnaviridae family. It contains a positive, single-stranded RNA genome having two untranslated regions at the 5' and 3' ends, and a large open reading frame encoding for a 3,010 to 3,030 amino acid polyprotein [13Gerlich WH, Robinson WS. Hepatitis B virus contains protein attached to the 5′ terminus of its complete DNA strand. Cell 1980; 21(3): 801-9.
[http://dx.doi.org/10.1016/0092-8674(80)90443-2] [PMID: 7438207] ].
3.1. Mode of Transmission and Replication Cycle
Contaminated food or water acts as a source for spreading Hepatitis A and E. Transmission of hepatitis B, C and D generally takes place through the infected body fluids. These viruses are usually transmitted through transfusion of infected blood, use of contaminated equipment during surgery, and sexual contact. HBV is also transmitted from mother to child during parturition. Acute infection may be symptomatic or non-symptomatic. Symptoms include yellow colouration of eyes, skin and urine, intense weakness, abdominal pain, nausea, and vomiting [14Report WHO. 2016.What is Hepatitis? ]. The HBV infection involves an initial step that is attachments of mature virion to the host cell surface, with the help of preS domain of the surface protein [15Klingmüller U, Schaller H. Hepadnavirus infection requires interaction between the viral pre-S domain and a specific hepatocellular receptor. J Virol 1993; 67(12): 7414-22.
[PMID: 8230462] ]. Various factors have been suggested to act as receptors for viruses in the cell. However, only carboxypeptidase D mediated viral entry has been revealed during duck HBV infection [16Breiner KM, Urban S, Schaller H. Carboxypeptidase D (gp180), a Golgi-resident protein, functions in the attachment and entry of avian hepatitis B viruses. J Virol 1998; 72(10): 8098-104.
[PMID: 9733850] ]. Disassembly of the virus and mechanism of intracellular nuclear transport for the viral genome are not clearly understood and nucleocapsid core protein modification has been implicated in the process [17Kang HY, Lee S, Park SG, Yu J, Kim Y, Jung G. Phosphorylation of hepatitis B virus Cp at Ser87 facilitates core assembly. Biochem J 2006; 398(2): 311-7.
[http://dx.doi.org/10.1042/BJ20060347] [PMID: 16740137] ]. After nuclear import viral DNA is converted to the covalently closed circular DNA (cccDNA) (Fig. 2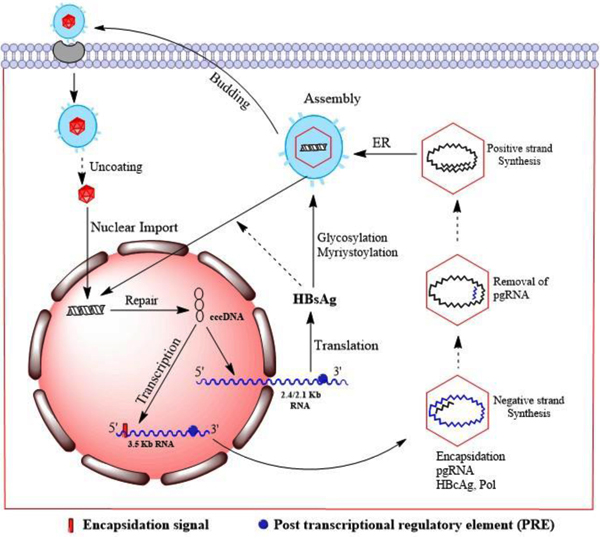 ) [18Beck J, Nassal M. Hepatitis B virus replication. World J Gastroenterol 2007; 13(1): 48-64.
) [18Beck J, Nassal M. Hepatitis B virus replication. World J Gastroenterol 2007; 13(1): 48-64.
[http://dx.doi.org/10.3748/wjg.v13.i1.48] [PMID: 17206754] ]. The cccDNA transcripts do not undergo splicing and have a polyadenylated structure with a 5' cap. Two different 5' ends are present in the genomic transcripts (3.5kb) which consist of two species i.e., the pregenomic RNA (pgRNA) and the precore RNA. The pgRNA serves as messenger RNA for core and polymerase as well as the template for reverse transcription. The pre-core RNA is translated into the pre-core gene products. Through ribosomal scanning mechanism of the pgRNA, the pol start codon initiates the polymerase translation [19Jean-Jean O, Weimer T, de Recondo AM, Will H, Rossignol JM. Internal entry of ribosomes and ribosomal scanning involved in hepatitis B virus P gene expression. J Virol 1989; 63(12): 5451-4.
[PMID: 2585611] ]. The 2.4kb subgenomic RNA produces large HBsAg protein, 2.1kb RNAs produce the middle HBsAg (M-HBsAg) and small HBsAg (S-HBsAg) proteins, and 0.7kb RNA is translated to the HBxAg protein.
 |
Fig. (2) Replication cycle of HBV. |
4. MOLECULAR MECHANISM OF HEPATOCELLULAR CARCINOMA
HCC is the outcome of many variable etiological factors such as HBV, HCV, alcohol, aflatoxins, inborn and acquired metabolic diseases. The carcinoma might originate in mature liver cells or progenitor cell. Hence, the molecular basis of HCC progression may differ depending on diverse factors and therefore, a number of mechanisms might be involved [20Alotaibi H, Atabey N, Diril N, Erdal E, Ozturk M. Molecular mechanism of hepatocellular carcinoma.Hepatocellular Carcinoma 2016; 43-63. [http://dx.doi.org/ 10.1007/978-3-319-34214-6_3]
[http://dx.doi.org/10.1007/978-3-319-34214-6_3] ]. Some important mechanisms associated with the hepatocellular carcinogenesis are described below.
4.1. Loss of Cell Cycle Control
Loss of cell cycle control is a general feature observed in all cancerous cells. This leads to an increased multiplicative tendency, hyperplasia, and subsequent tumour formation. Normal liver cells primarily live in the G0 phase (quiescent phase) of the cell cycle and renewed slowly. However, they possess the strong regenerative ability and after getting mitogenic signals, they enter the cell cycle and proceed to cell division [21Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 2010; 176(1): 2-13.
[http://dx.doi.org/10.2353/ajpath.2010.090675] [PMID: 20019184] ]. Advancement through the eukaryotic cell cycle phases is governed by the combined actions of cyclins and cyclin-dependent kinases (Cdk). Cytokines and growth factors promote de-novo expression of cyclin D1 gene which is responsible for the transition of quiescent hepatocytes into the cell cycle [22Sharma AK, Kumar S, Chashoo G, Saxena AK, Pandey AK. Cell cycle inhibitory activity of Piper longum against A549 cell line and its protective effect against metal-induced toxicity in rats. Ind J Biochem Biophys 2014; 51(5): 358-64.
[PMID: 25630105] , 23Boylan JM, Gruppuso PA. D-type cyclins and G1 progression during liver development in the rat. Biochem Biophys Res Commun 2005; 330(3): 722-30.
[http://dx.doi.org/10.1016/j.bbrc.2005.03.042] [PMID: 15809057] ]. Many regulatory checkpoints apply the brake on free proliferation and avert quiescent hepatocytes from entry in the cell cycle (Fig. 3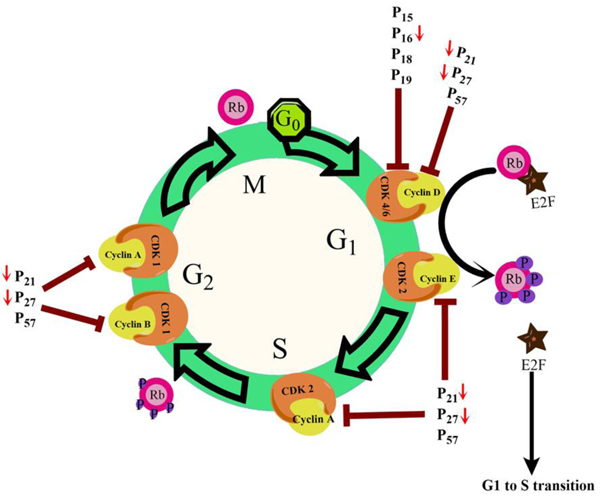 ). As depicted in Fig. (3
). As depicted in Fig. (3 ), retinoblastoma (pRb) and other proteins bind to and seize E2F transcription factors and thereby repress its activity [24Henley SA, Dick FA. The retinoblastoma family of proteins and their regulatory functions in the mammalian cell division cycle. Cell Div 2012; 7(1): 10.
), retinoblastoma (pRb) and other proteins bind to and seize E2F transcription factors and thereby repress its activity [24Henley SA, Dick FA. The retinoblastoma family of proteins and their regulatory functions in the mammalian cell division cycle. Cell Div 2012; 7(1): 10.
[http://dx.doi.org/10.1186/1747-1028-7-10] [PMID: 22417103] ]. Entry into the cell cycle is also prevented by the Ink4 family of Cdk inhibitors (p15/16/18/19) by binding to Cdk4/6 kinases and inhibiting the formation of cyclin D-Cdk4/6 complex [25Ortega S, Malumbres M, Barbacid M. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim Biophys Acta 2002; 1602(1): 73-87.
[PMID: 11960696] ]. Binding of CDK interacting protein (Cip)/Kinase inhibitory protein (Kip)family inhibitory proteins p21/27/57 with Cdk/cyclin complexes inactivates it and inhibits cell cycle advancement [26Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell 2008; 14(2): 159-69.
[http://dx.doi.org/10.1016/j.devcel.2008.01.013] [PMID: 18267085] ]. A “proliferation cluster” has been identified in gene expression profiles of HCC samples which accounted for increased expression of proliferation-associated genes [27Chen X, Cheung ST, So S, et al. Gene expression patterns in human liver cancers. Mol Biol Cell 2002; 13(6): 1929-39.
[http://dx.doi.org/10.1091/mbc.02-02-0023] [PMID: 12058060] , 28Xu XR, Huang J, Xu ZG, et al. Insight into hepatocellular carcinogenesis at transcriptome level by comparing gene expression profiles of hepatocellular carcinoma with those of corresponding noncancerous liver. Proc Natl Acad Sci USA 2001; 98(26): 15089-94.
[http://dx.doi.org/10.1073/pnas.241522398] [PMID: 11752456] ]. Abnormalities that decrease the expression levels of p16/pRb genes or hamper their protein functions eventually cause tumorigenesis because p16/pRb pathway manages entry into cell cycle. Altered expression of the pRb is a universal phenomenon in HCC [29Murakami Y, Saigo K, Takashima H, et al. Large scaled analysis of hepatitis B virus (HBV) DNA integration in HBV related hepatocellular carcinomas. Gut 2005; 54(8): 1162-8.
[http://dx.doi.org/10.1136/gut.2004.054452] [PMID: 16009689] ]. It has been reported that the expression levels of CIP/KIP family member proteins p21/27 are frequently reduced in HCC samples [30Tannapfel A, Grund D, Katalinic A, et al. Decreased expression of p27 protein is associated with advanced tumor stage in hepatocellular carcinoma. Int J Cancer 2000; 89(4): 350-5.
[http://dx.doi.org/10.1002/1097-0215(20000720)89:4<350::AID-IJC6>3.0.CO;2-3] [PMID: 10956409] ].
4.2. Loss of Senescence Control
Senescence is a type of irreversible growth inhibition of cells in cell culture showing distinct morphological alterations [31Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 1965; 37(3): 614-36.
[http://dx.doi.org/10.1016/0014-4827(65)90211-9] [PMID: 14315085] ]. In hepatocytes, mechanism of senescence is not clearly understood. Replicative senescence controls partial proliferative ability of liver cells by a gradual decrease in the telomeric segment [32Paradis V, Youssef N, Dargère D, et al. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol 2001; 32(3): 327-32.
[http://dx.doi.org/10.1053/hupa.2001.22747] [PMID: 11274643] ]. Telomere-independent mechanisms have also been suggested for hepatocyte senescence monitored in severe chronic liver diseases and these include free radical and oncogene-dependent senescence The resulting DNA damage activates ATM/Chk/p53 pathway and arrests cells at G1 phase. Alternatively, the p16/pRb pathway also performs the same function. Anomalies in DNA damage checkpoint and cell cycle regulatory pathway paved a way for the unlimited proliferation of genetically altered hepatic cells at the senescent phase and subsequently to malignant transformation. (Fig. 4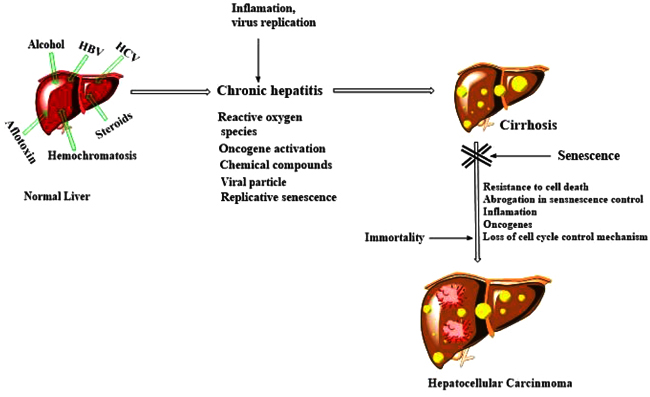 ).
).
 |
Fig. (4) The proposed model of hepatocellular carcinoma development. |
In human HCC, the p53 pathway has an effect on many levels i.e., (a) about 50% aflatoxin-mediated HCC cases exhibit p53 mutations while 20–30% cases of non-aflatoxin mediated HCC show p53 mutations; (b) microdeletions of p14ARF rarely occurs in HCC with p53 mutation while it is reported in 15-20% of human HCC; (c) human HCC also shows elevated Mdm2 expression; (d) over expression of gankyrin, an oncoprotein, is commonly observed in human HCC, which imposes restriction on the pRb and p53 [33Higashitsuji H, Higashitsuji H, Itoh K, et al. The oncoprotein gankyrin binds to MDM2/HDM2, enhancing ubiquitylation and degradation of p53. Cancer Cell 2005; 8(1): 75-87.
[http://dx.doi.org/10.1016/j.ccr.2005.06.006] [PMID: 16023600] ].
pRb pathway anomalies (p16, p15 or RB1 genes) are observed in more than 80% of human HCC. The anomalies include p16/15 promoter methylation and deletion or mutation of RB1 gene. Promoter methylation causing p16 repression is the most common anomaly [34Azechi H, Nishida N, Fukuda Y, et al. Disruption of the p16/cyclin D1/retinoblastoma protein pathway in the majority of human hepatocellular carcinomas. Oncology 2001; 60(4): 346-54.
[http://dx.doi.org/10.1159/000058531] [PMID: 11408803] ]. Telomerase activation occurs during the transformation of precancerous lesions to HCC. Telomere-dependent senescence arrest in hepatocytes is frequently observed in cirrhosis. Reactivation of Telomerase Reverse Transcriptase (TERT) acts as a bypass for HCC growth. TERT is absent in normal hepatocytes, hence 90% human HCC show telomerase activation, a rate-limiting step for the commencement of cell immortality [35Llovet JM, Chen Y, Wurmbach E, et al. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology 2006; 131(6): 1758-67.
[http://dx.doi.org/10.1053/j.gastro.2006.09.014] [PMID: 17087938] ]. Deregulation of TERT expression by integration of HBV DNA into TERT gene is a rare phenomenon [29Murakami Y, Saigo K, Takashima H, et al. Large scaled analysis of hepatitis B virus (HBV) DNA integration in HBV related hepatocellular carcinomas. Gut 2005; 54(8): 1162-8.
[http://dx.doi.org/10.1136/gut.2004.054452] [PMID: 16009689] ]. Besides, HBV surface proteins (viral X and PreS2) and HCV core protein may increase the activity of telomerase [36Liu H, Luan F, Ju Y, et al. In vitro transfection of the hepatitis B virus PreS2 gene into the human hepatocarcinoma cell line HepG2 induces upregulation of human telomerase reverse transcriptase. Biochem Biophys Res Commun 2007; 355(2): 379-84.
[http://dx.doi.org/10.1016/j.bbrc.2007.01.160] [PMID: 17307151] ]. The above-mentioned facts indicate the cooperation between the anomalies in telomerase activity and senescence controlling genes (p53) during the hepatocarcinogenesis.
4.3. Dysregulation of Apoptosis
Cell death resulting from liver injury may be either accidental (necrotic), programmed (apoptotic), or uncontrolled. Extrinsic or intrinsic pathways initiate apoptosis by activating caspases 3, 6 and 7 [22Sharma AK, Kumar S, Chashoo G, Saxena AK, Pandey AK. Cell cycle inhibitory activity of Piper longum against A549 cell line and its protective effect against metal-induced toxicity in rats. Ind J Biochem Biophys 2014; 51(5): 358-64.
[PMID: 25630105] , 37Eguchi A, Wree A, Feldstein AE. Biomarkers of liver cell death. J Hepatol 2014; 60(5): 1063-74.
[http://dx.doi.org/10.1016/j.jhep.2013.12.026] [PMID: 24412608] ]. Death receptors mediate resistance towards apoptosis in HCC cells. The majority of the HCCs show one or more alterations in the Fas pathway molecules, which inhibit Fas-mediated apoptosis. HCC cells or tissues become unresponsive to Fas by downregulating Fas expression resulting in reduced expression of FADD or FLICE or increased expression of cellular FLICE/caspase-8-inhibitory protein (cFLIP), or by upregulation of nuclear factor-kappa B (NF-κB), Bcl-2 or Bcl-XL and Mcl-1 [38Okano H, Shiraki K, Inoue H, et al. Cellular FLICE/caspase-8-inhibitory protein as a principal regulator of cell death and survival in human hepatocellular carcinoma. Lab Invest 2003; 83(7): 1033-43.
[http://dx.doi.org/10.1097/01.LAB.0000079328.76631.28] [PMID: 12861043] -40Ranjan K, Pathak C. FADD regulates NF-κB activation and promotes ubiquitination of cFLIPL to induce apoptosis. Sci Rep 2016; 6: 22787.
[http://dx.doi.org/10.1038/srep22787] [PMID: 26972597] ]. Pro-apoptotic proteins (Bax or Bcl-XS) are downregulated in HCC. The TGF-β pathway is regularly stimulated at the cirrhosis stage and promotes apoptosis by activating Smad3 mediated Bcl2 downregulation and thereby reducing the susceptibility towards HCC development [41Yang YA, Zhang GM, Feigenbaum L, Zhang YE. Smad3 reduces susceptibility to hepatocarcinoma by sensitizing hepatocytes to apoptosis through downregulation of Bcl-2. Cancer Cell 2006; 9(6): 445-57.
[http://dx.doi.org/10.1016/j.ccr.2006.04.025] [PMID: 16766264] ]. Insulin-receptor signalling and activation of the PI3K-Akt pathway might also be involved in resistance towards apoptosis [42Chen RH, Su YH, Chuang RL, Chang TY. Suppression of transforming growth factor-beta-induced apoptosis through a phosphatidylinositol 3-kinase/Akt-dependent pathway. Oncogene 1998; 17(15): 1959-68.
[http://dx.doi.org/10.1038/sj.onc.1202111] [PMID: 9788439] ]. The insulin-like growth factor 2 receptor (IGF2R) reduces cell division by stimulating TGF-β signalling and breakdown of the IGF2 mitogen [43Dennis PA, Rifkin DB. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci USA 1991; 88(2): 580-4.
[http://dx.doi.org/10.1073/pnas.88.2.580] [PMID: 1846448] ]. During the initial phase of human hepatocarcinogenesis heterozygosity in IGF2R locus is frequently lost [44Yamada T, De Souza AT, Finkelstein S, Jirtle RL. Loss of the gene encoding mannose 6-phosphate/insulin-like growth factor II receptor is an early event in liver carcinogenesis. Proc Natl Acad Sci USA 1997; 94(19): 10351-5.
[http://dx.doi.org/10.1073/pnas.94.19.10351] [PMID: 9294214] ]. In human HCCs loss of IGF2R and overexpression of IGF2 growth factor are common features. Stimulation of the Akt signalling and reduced expression of a negative regulator of Akt i.e., phosphatase and tensin homolog (PTEN) have been described in 40-60% HCC cases [45Hu TH, Huang CC, Lin PR, et al. Expression and prognostic role of tumor suppressor gene PTEN/MMAC1/TEP1 in hepatocellular carcinoma. Cancer 2003; 97(8): 1929-40.
[http://dx.doi.org/10.1002/cncr.11266] [PMID: 12673720] ].
4.4. Liver Inflammation and Hepatocarcinogenesis
Most of the studies suggest that liver injury in viral hepatitis does not result from the direct cytopathic effects of viruses but caused by the viral protein-mediated host immune response [46Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol 1995; 13: 29-60.
[http://dx.doi.org/10.1146/annurev.iy.13.040195.000333] [PMID: 7612225] ]. Animal studies have provided ample proof that viral hepatitis is triggered by an antigen-specific intrahepatic cellular response that set in motion a series of antigen-nonspecific cellular and molecular effector systems. Cellular and humoral limbs of the immune system work towards viral clearance by three different mechanisms: firstly, the virus-specific T-cell mediated direct destruction of infected hepatocytes; secondly, the removal of free viral particles from the circulation by the antibody response; and thirdly, non-cytopathic viral inactivation in infected hepatocytes by some inflammatory cytokines produced by activated mononuclear cells [47Ferrari C, Chisari FV. In: The liver biology and pathobiology. Arias, I.M., (ed.), Lippincott Williams & Wilkins, Philadelphia. 2001; pp. 763-82.]. Recent evidence suggests that NF-κB signalling mediated inflammation plays an essential role in commencement, promotion and development of tumours [48Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev 2008; 18(1): 19-26.
[http://dx.doi.org/10.1016/j.gde.2008.01.020] [PMID: 18440219] ].
4.4.1. Cytokines
Various inflammatory cytokines viz., interleukin-1α (IL-1α), IL-1β, IL-6, IL-8 and tumour necrosis factor-α (TNF-α), participate in chronic hepatic inflammation. Among these, IL-6 is the most important and is produced by activated kupffer cells in chronic hepatitis. It results in local inflammatory response and activates hepatocyte proliferation leading to cancerous hepatocytes [49Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med 2008; 14(3): 109-19.
[http://dx.doi.org/10.1016/j.molmed.2007.12.007] [PMID: 18261959] ]. In chronic liver diseases such as HBV and HCV induced hepatitis, alcoholic hepatitis and non-alcoholic steatohepatitis increased serum IL-6 levels have been observed. These reports highlight the vital role played by IL-6 in human hepatocarcinogenesis. IL-6 knockout mice exhibited a significant reduction of diethylnitrosamine (DENA)-initiated HCC development, suggesting a direct involvement of IL-6 signalling in experimental hepatocarcinogenesis. Role of innate immune response in the hepatocarcinogenesis has also been demonstrated by IL-6 production via stimulation of Toll-Like Receptor (TLR) mediated through MyD88in rodents [50Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007; 317(5834): 121-4.
[http://dx.doi.org/10.1126/science.1140485] [PMID: 17615358] ].
4.4.2. NF-κB Pathway
NF-κB, a transcription factor, plays a key role in innate immunity and liver inflammatory signalling pathways [51Muriel P. NF-kappaB in liver diseases: A target for drug therapy. J Appl Toxicol 2009; 29(2): 91-100.
[http://dx.doi.org/10.1002/jat.1393] [PMID: 18937212] , 52Xiao C, Ghosh S. NF-kappaB, an evolutionarily conserved mediator of immune and inflammatory responses. Adv Exp Med Biol 2005; 560: 41-5.
[http://dx.doi.org/10.1007/0-387-24180-9_5] [PMID: 15932018] ]. It is activated by cytokines or interleukins such as TNF-α, IL-6 and IL-1β, viral and bacterial DNA and RNA and pathogen-derived lipopolysaccharides. NF-κB dimer formed after activation undergoes nuclear translocation, attaches with particular DNA segment, and triggers transcription of genes related to immune responses, inflammation, proliferation and survival of cells [53Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999; 18(49): 6853-66.
[http://dx.doi.org/10.1038/sj.onc.1203239] [PMID: 10602461] , 54Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell 2002; 109(Suppl.): S81-96.
[http://dx.doi.org/10.1016/S0092-8674(02)00703-1] [PMID: 11983155] ]. In all chronic liver diseases viz., alcoholic/non-alcoholic/biliary liver disease and viral hepatitis NF-κB gets activated [55Luedde T, Schwabe RF. NF-κB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2011; 8(2): 108-18.
[http://dx.doi.org/10.1038/nrgastro.2010.213] [PMID: 21293511] ]. It has been demonstrated that inducible IκB super-repressor mediated NF-κB inhibition reduced hepatic tumour development in chronic inflammation induced Mdr2 knockout mouse, the animal HCC model [56Mauad TH, van Nieuwkerk CM, Dingemans KP, et al. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol 1994; 145(5): 1237-45.
[PMID: 7977654] , 57Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004; 431(7007): 461-6.
[http://dx.doi.org/10.1038/nature02924] [PMID: 15329734] ]. The liver tumour-promoting activity of NF-κB has been validated in another inflammatory HCC model i.e., hepatocyte-specific lymphotoxin αβ transgenic mouse model. In this model, NF-κB was inhibited by hepatocyte-specific deletion of IKK-β which resulted in entirely reduced HCC progression [58Haybaeck J, Zeller N, Wolf MJ, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell 2009; 16(4): 295-308.
[http://dx.doi.org/10.1016/j.ccr.2009.08.021] [PMID: 19800575] ].
5. Characterisation of Hepatocellular Carcinoma: Biomarkers
With the vast input of knowledge about tumour biology, curiosity for identifying HCC related molecular markers has increased. During the new era of “omics”, the emergence of a number of cutting-edge technologies such as next-generation sequencing and microarray has advanced the search for biomarkers [59Aravalli RN, Steer CJ, Cressman EN. Molecular mechanisms of hepatocellular carcinoma. Hepatology 2008; 48(6): 2047-63.
[http://dx.doi.org/10.1002/hep.22580] [PMID: 19003900] -61Cho W, Ziogas DE, Katsios C, Roukos DH. Emerging personalized oncology: sequencing and systems strategies. Future Oncol 2012; 8(6): 637-41.
[http://dx.doi.org/10.2217/fon.12.44] [PMID: 22764759] ]. These technologies have given an advantage in examining the tumour genome (single nucleotide polymorphism, variations in copy number, aneuploidy and loss of heterogeneity), transcriptome, proteome, epigenome, metabolome, and miRNA profile [62Budhu A, Ji J, Wang XW. The clinical potential of microRNAs. J Hematol Oncol 2010; 3: 37.
[http://dx.doi.org/10.1186/1756-8722-3-37] [PMID: 20925959] -64You JS, Jones PA. Cancer genetics and epigenetics: Two sides of the same coin? Cancer Cell 2012; 22(1): 9-20.
[http://dx.doi.org/10.1016/j.ccr.2012.06.008] [PMID: 22789535] ]. Currently, several markers in blood and tissue have been identified [65Ijichi M, Takayama T, Matsumura M, Shiratori Y, Omata M, Makuuchi M. alpha-Fetoprotein mRNA in the circulation as a predictor of postsurgical recurrence of hepatocellular carcinoma: A prospective study. Hepatology 2002; 35(4): 853-60.
[http://dx.doi.org/10.1053/jhep.2002.32100] [PMID: 11915031] , 66Sund M, Kalluri R. Tumor stroma derived biomarkers in cancer. Cancer Metastasis Rev 2009; 28(1-2): 177-83.
[http://dx.doi.org/10.1007/s10555-008-9175-2] [PMID: 19259624] ]. A detailed account of various HCC markers is given below.
5.1. Metabolic Biomarkers
5.1.1. α-Fetoprotein
Since the discovery of α-fetoprotein (AFP) in the serum of HCC patients, AFP is considered as the most important biomarker for assessment of HCC [67Tatarinov IuS. Detection of embryo-specific alpha-globulin in the blood serum of a patient with primary liver cancer. Vopr Med Khim 1964; 10: 90-1.
[PMID: 14207501] ]. It is a glycoprotein (MW 70 kDa) responsible for transport of several compounds viz., steroids, bilirubin, retinoid, fatty acids, flavonoids, heavy metals, dioxin, dyes, phytoestrogens, drugs etc [68Mizejewski GJ. Alpha-fetoprotein structure and function: relevance to isoforms, epitopes, and conformational variants. Exp Biol Med (Maywood) 2001; 226(5): 377-408.
[http://dx.doi.org/10.1177/153537020122600503] [PMID: 11393167] ]. It is produced by the fetal liver, yolk sac and intestine during development [69Gitlin D, Perricelli A, Gitlin JD. The presence of serum alpha-fetoprotein in sharks and its synthesis by fetal gastrointestinal tract and liver. Comp Biochem Physiol B 1973; 46(2): 207-15.
[http://dx.doi.org/10.1016/0305-0491(73)90311-8] [PMID: 4127981] ]. During 12-16 weeks of fetal development, AFP in serum reaches the highest concentration (3 g/L). Subsequently, there is a rapid decline in the levels and only traces are detectable in serum [70Debruyne EN, Delanghe JR. Diagnosing and monitoring hepatocellular carcinoma with alpha-fetoprotein: new aspects and applications. Clin Chim Acta 2008; 395(1-2): 19-26.
[http://dx.doi.org/10.1016/j.cca.2008.05.010] [PMID: 18538135] ]. Unusually elevated serum AFP levels find a correlation with the malignant diseases including HCC [71Chayvialle JA, Ganguli PC. Radioimmunoassay of alpha-fetoprotein in human plasma. Lancet 1973; 1(7816): 1355-7.
[http://dx.doi.org/10.1016/S0140-6736(73)91676-0] [PMID: 4122743] , 72Waldmann TA, McIntire KR. The use of a radioimmunoassay for alpha-fetoprotein in the diagnosis of malignancy. Cancer 1974; 34(4)(Suppl.): 1510-5.
[http://dx.doi.org/10.1002/1097-0142(197410)34:8+<1510::AID-CNCR2820340824>3.0.CO;2-Y] [PMID: 4138906] ]. AFP is found in three glycoforms based on lectin binding pattern i.e., the non-binding fraction AFP-L1, the weak binding fraction AFP-L2, and the binding fraction AFP-L3. Liver cirrhosis and chronic hepatitis show elevated levels of AFP-L1, whereas in HCC AFP-L3 is notably increased. Only cancer cells produce AFP-L3 hence, it is regarded as specific HCC biomarker [73Sato Y, Nakata K, Kato Y, et al. Early recognition of hepatocellular carcinoma based on altered profiles of alpha-fetoprotein. N Engl J Med 1993; 328(25): 1802-6.
[http://dx.doi.org/10.1056/NEJM199306243282502] [PMID: 7684823] , 74Spangenberg HC, Thimme R, Blum HE. Serum markers of hepatocellular carcinoma. Semin Liver Dis 2006; 26(4): 385-90.
[http://dx.doi.org/10.1055/s-2006-951606] [PMID: 17051452] ].
5.1.2. Des-γ-Carboxyprothrombin (DCP)
Des-γ-carboxyprothrombin (DCP) is an abnormal form of prothrombin and also called prothrombin induced by vitamin K absence-II (PIVKA II). The production of DCP stems from a defective vitamin K-dependent posttranslational carboxylation system, which induces the malignant transformation of HCC cells [75Naraki T, Kohno N, Saito H, et al. gamma-Carboxyglutamic acid content of hepatocellular carcinoma-associated des-gamma-carboxy prothrombin. Biochim Biophys Acta 2002; 1586(3): 287-98.
[http://dx.doi.org/10.1016/S0925-4439(01)00107-7] [PMID: 11997080] ]. Normal prothrombin function of DCP is lost and it supports malignant growth in HCC. Serum DCP levels in patients (benign and malignant liver diseases) varies considerably. Its sensitivity as a diagnostic agent might be better than AFP. This result still needs validation [76Volk ML, Hernandez JC, Su GL, Lok AS, Marrero JA. Risk factors for hepatocellular carcinoma may impair the performance of biomarkers: a comparison of AFP, DCP, and AFP-L3. Cancer Biomark 2007; 3(2): 79-87.
[http://dx.doi.org/10.3233/CBM-2007-3202] [PMID: 17522429] ].
5.1.3. Glypican-3
Glypican-3 (GPC3) belongs to the glypican family of heparan sulfate proteoglycans. Glycosyl-phosphatidylinositol anchor links it to the cell membrane [77Filmus J. The contribution of in vivo manipulation of gene expression to the understanding of the function of glypicans. Glycoconj J 2002; 19(4-5): 319-23.
[http://dx.doi.org/10.1023/A:1025312819804] [PMID: 12975611] ]. GPC3 is responsible for cell proliferation, survival, and tumour suppression, but is normally absent in healthy and non-malignant hepatocytes. GPC3 acts as a biomarker for different types of cancers. It is upregulated in HCC whereas it is downregulated in lung adenocarcinoma, ovarian cancer, and breast cancer [78Filmus J, Capurro M. The role of glypican-3 in the regulation of body size and cancer. Cell Cycle 2008; 7(18): 2787-90.
[http://dx.doi.org/10.4161/cc.7.18.6672] [PMID: 18787398] , 79Sung YK, Hwang SY, Park MK, et al. Glypican-3 is overexpressed in human hepatocellular carcinoma. Cancer Sci 2003; 94(3): 259-62.
[http://dx.doi.org/10.1111/j.1349-7006.2003.tb01430.x] [PMID: 12824919] ]. In HCC, it has been suggested to act as growth stimulator by upregulating autocrine/paracrine canonical Wnt signalling [80Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res 2005; 65(14): 6245-54.
[http://dx.doi.org/10.1158/0008-5472.CAN-04-4244] [PMID: 16024626] ].
5.1.4. Golgi Protein-73 (GP73)
GP73 (MW 73kDa) is present in the Golgi complex as a transmembrane glycoprotein. It is expressed in normal biliary epithelial cells whereas it is not expressed in normal hepatocytes. In hepatic diseases such as HCC, its expression is considerably enhanced [81Kladney RD, Cui X, Bulla GA, Brunt EM, Fimmel CJ. Expression of GP73, a resident Golgi membrane protein, in viral and nonviral liver disease. Hepatology 2002; 35(6): 1431-40.
[http://dx.doi.org/10.1053/jhep.2002.32525] [PMID: 12029628] ]. It has been reported that serum GP73 in HCC patients was appreciably greater than in normal healthy persons and HBV carriers [82Mao Y, Yang H, Xu H, et al. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut 2010; 59(12): 1687-93.
[http://dx.doi.org/10.1136/gut.2010.214916] [PMID: 20876776] ].
5.1.5. Squamous Cell Carcinoma Antigen (SCCA)
Squamous Cell Carcinoma Antigen (SCCA) belongs to the family of serine protease inhibitors found in squamous epithelium and in cervical carcinoma. Epithelial tumours exhibit higher expression of SCCA and act as an anti-apoptotic agent [83Suminami Y, Kishi F, Sekiguchi K, Kato H. Squamous cell carcinoma antigen is a new member of the serine protease inhibitors. Biochem Biophys Res Commun 1991; 181(1): 51-8.
[http://dx.doi.org/10.1016/S0006-291X(05)81380-4] [PMID: 1958219] ]. Dedifferentiation results in SCCA expression and it is considered as a prospective HCC biomarker. It has been reported that HCC patients showed higher serum SCCA levels than patients with cirrhosis [84Giannelli G, Fransvea E, Trerotoli P, et al. Clinical validation of combined serological biomarkers for improved hepatocellular carcinoma diagnosis in 961 patients. Clin Chim Acta 2007; 383(1-2): 147-52.
[http://dx.doi.org/10.1016/j.cca.2007.05.014] [PMID: 17582392] ]. An alternative prospective marker is the SCCA complexed with IgM (SCCA-IgM). During the early phase of hepatocarcinogenesis, its expression is increased. Reports based on serum samples collected from HCC/cirrhosis patients and healthy volunteers, SCCA-IgM got a higher sensitivity value than AFP, but a lower specificity in HCC diagnosis. Therefore, SCCA-IgM may be an important serum biomarker for early detection of HCC [85Pozzan C, Cardin R, Piciocchi M, et al. Diagnostic and prognostic role of SCCA-IgM serum levels in hepatocellular carcinoma (HCC). J Gastroenterol Hepatol 2014; 29(8): 1637-44.
[http://dx.doi.org/10.1111/jgh.12576] [PMID: 24635038] ].
5.2. Genetic and Epigenetic Events in HCC
HCC initiation and progression is associated with genetic alteration. The permanent genetic abnormalities build up in hepatocytes and cause disrupted gene expression which ultimately leads to cancerous transformation. Genetic alterations include large chromosomal translocation, amplification, single nucleotide variation, small fraction loss and deletion. The genetic changes frequently cause the loss of function or activation of oncogenes or tumour suppressor genes. Contrary to genetic alterations, no change in the genome sequence is found in epigenetic regulations but it influences the chromatin structure and transcription of the gene. Gene products are affected at transcriptional and post-transcriptional levels during epigenetic regulations which include DNA methylation, histone modification, and lncRNA. This provides greater diversity to the gene regulation [86Singh AK, Bishayee A, Pandey AK. Targeting histone deacetylases with natural and synthetic agent: An emerging anticancer strategy. Nutrients 2018; 10(6): 731.
[http://dx.doi.org/10.3390/nu10060731] [PMID: 29882797] ].
5.2.1. Chromosomal Instability
In HCC, chromosomal instability is the most frequently observed genetic changes. It could be promoted by either error during mitosis or disruption in DNA replication and repair processes. The chromosome abnormalities include amplification/deletion of small chromosomal segments or gain/loss of whole chromosome arms. Comparative genomic hybridization data in HCC represent frequent amplification of chromosome 1q and 8q, while the frequent loss of chromosome 1p, 4q, 6q, 9p, 16p, 16q, and 17p (Table 1) [87Guan XY, Fang Y, Sham JST, et al. Recurrent chromosome alterations in hepatocellular carcinoma detected by comparative genomic hybridization. Genes Chromosomes Cancer 2000; 29(2): 110-6.
[http://dx.doi.org/10.1002/1098-2264(2000)9999:9999<::AID-GCC1022>3.0.CO;2-V] [PMID: 10959090] ]. Chromosome 1q amplification in HCC is a characteristic feature of chromosome abnormalities. In a large number of HCC patients, the chromosome 1q21 region containing CHD1L (an oncogene) was reported to be amplified [110Ma NF, Hu L, Fung JM, et al. Isolation and characterization of a novel oncogene, amplified in liver cancer 1, within a commonly amplified region at 1q21 in hepatocellular carcinoma. Hepatology 2008; 47(2): 503-10.
[http://dx.doi.org/10.1002/hep.22072] [PMID: 18023026] ]. CHD1L is associated with oncogenic functions during hepatocarcinogenesis such as anti-apoptotic, mitosis regulation, and stimulating cell epithelial-to-mesenchymal transition [88Chen L, Chan THM, Yuan YF, et al. CHD1L promotes hepatocellular carcinoma progression and metastasis in mice and is associated with these processes in human patients. J Clin Invest 2010; 120(4): 1178-91.
[http://dx.doi.org/10.1172/JCI40665] [PMID: 20335658] , 111Chen L, Hu L, Chan THM, et al. Chromodomain helicase/adenosine triphosphatase DNA binding protein 1-like (CHD1l) gene suppresses the nucleus-to-mitochondria translocation of nur77 to sustain hepatocellular carcinoma cell survival. Hepatology 2009; 50(1): 122-9.
[http://dx.doi.org/10.1002/hep.22933] [PMID: 19441106] ]. In HCC chromosome, 8q24 region is another highly amplified region which contains oncogenes including c-Myc and PTK2 [98Santoni-Rugiu E, Jensen MR, Thorgeirsson SS. Disruption of the pRb/E2F pathway and inhibition of apoptosis are major oncogenic events in liver constitutively expressing c-myc and transforming growth factor alpha. Cancer Res 1998; 58(1): 123-34.
[PMID: 9426068] , 99Okamoto H, Yasui K, Zhao C, Arii S, Inazawa J. PTK2 and EIF3S3 genes may be amplification targets at 8q23-q24 and are associated with large hepatocellular carcinomas. Hepatology 2003; 38(5): 1242-9.
[http://dx.doi.org/10.1053/jhep.2003.50457] [PMID: 14578863] , 112Wang Y, Wu MC, Sham JS, Zhang W, Wu WQ, Guan XY. Prognostic significance of c-myc and AIB1 amplification in hepatocellular carcinoma. A broad survey using high-throughput tissue microarray. Cancer 2002; 95(11): 2346-52.
[http://dx.doi.org/10.1002/cncr.10963] [PMID: 12436441] ]. SGK3A, a serine/threonine kinase, having similarity with AKT is commonly amplified in HCC which provides AKT independent oncogenic roles [100Liu M, Chen L, Chan THM, et al. Serum and glucocorticoid kinase 3 at 8q13.1 promotes cell proliferation and survival in hepatocellular carcinoma. Hepatology 2012; 55(6): 1754-65.
[http://dx.doi.org/10.1002/hep.25584] [PMID: 22262416] ]. Segmental loss of chromosome 1p35-36 region containing many tumour suppressors (14-3-3 σ and Rb-interacting zinc finger 1) is also commonly found in HCC. Short arm loss of chromosome 8 (a minimal region of 8p21-22 containing DLC-1) is a common feature in HCC. Because of promoter hypermethylation and allele loss, DLC-1 is recurrently deleted in HCC tissues [113Wong CM, Lee JM, Ching YP, Jin DY, Ng IO. Genetic and epigenetic alterations of DLC-1 gene in hepatocellular carcinoma. Cancer Res 2003; 63(22): 7646-51.
[PMID: 14633684] ]. DLC-1 expression restoration in hepatoma cells could induce cell apoptosis, and inhibit tumour growth [114Zhou XL, Thorgeirsson SS, Popescu NC. Restoration of DLC-1 gene expression induces apoptosis and inhibits both cell growth and tumorigenicity in human hepatocellular carcinoma cells. Oncogene 2004; 23(6):1308-13. [https://doi.org/10.1038/sj.onc.1207246][PMID: 14647417].
5.2.2. Circulating miRNAs
MicroRNA (miR), a class of non-coding RNAs, has been identified as important regulators of gene expression at post-transcriptional levels. Role of circulating miRNAs in serum as cancer biomarkers were described in 2008 and overexpression of miR-155, miR-21 and miR-210 were observed in B-cell lymphoma patients [115Lawrie CH, Gal S, Dunlop HM, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol 2008; 141(5): 672-5.
[http://dx.doi.org/10.1111/j.1365-2141.2008.07077.x] [PMID: 18318758] ]. Abnormal expression of HCC development and progression related miRNAs and their role is under investigation. miR-122 and miR-221 regulate the cell cycle by modulating cyclins or cdk [116Gramantieri L, Ferracin M, Fornari F, et al. Cyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res 2007; 67(13): 6092-9.
[http://dx.doi.org/10.1158/0008-5472.CAN-06-4607] [PMID: 17616664] , 117Kumar S, Pandey AK. Oxidative stress-related microRNAs as diagnostic markers: A newer Insight in diagnostics. In: Maurya P, Chandra P. (eds.) Oxidative Stress: Diagnostic Methods and Applications in Medical Science. Springer, Singapore. 2017; 113-125.
[http://dx.doi.org/10.1007/978-981-10-4711-4_6] ]. Pro-apoptotic proteins (Bmf) are targets of some miRNAs (miR-221) which help HCC cells to avoid apoptosis [118Gramantieri L, Fornari F, Ferracin M, et al. MicroRNA-221 targets Bmf in hepatocellular carcinoma and correlates with tumor multifocality. Clin Cancer Res 2009; 15(16): 5073-81.
[http://dx.doi.org/10.1158/1078-0432.CCR-09-0092] [PMID: 19671867] ]. However, some miRNA (miR29) can promote HCC apoptosis by targeting the Bcl-2 and Mcl-1, the anti-apoptotic proteins [119Xiong Y, Fang JH, Yun JP, et al. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology 2010; 51(3): 836-45.
[PMID: 20041405] ]. The most important characteristics of HCC i.e., invasion and metastasis are also regulated by miRNAs. Cell migration and spreading in HCC is promoted by pro-metastatic miRNAs e.g., miR-106b induces cell migration and invasion in HCC by activating epithelial-mesenchymal transition process [120Yau WL, Lam CSC, Ng L, et al. Over-expression of miR-106b promotes cell migration and metastasis in hepatocellular carcinoma by activating epithelial-mesenchymal transition process. PLoS One 2013; 8(3): e57882.
[http://dx.doi.org/10.1371/journal.pone.0057882] [PMID: 23483935] ]. Metastasis and HCC progression are suppressed by let-7g, miR-139, and miR-195 [121Wang R, Zhao N, Li S, et al. MicroRNA-195 suppresses angiogenesis and metastasis of hepatocellular carcinoma by inhibiting the expression of VEGF, VAV2, and CDC42. Hepatology 2013; 58(2): 642-53.
[http://dx.doi.org/10.1002/hep.26373] [PMID: 23468064] ]. Unusually expressed miRNAs and their roles are given in Table 2.
5.2.3. Altered DNA Methylation Pattern
Abnormal DNA methylation is recurrently observed in human carcinomas. Methylation of cytosine residues in the promoter region takes place at CpG islands by DNA Methylase (DNMT). However, in tumour cells, the promoter methylation pattern is usually changed. Aberrant DNA methylation in the promoter regions of tumour suppressor genes results in transcriptional silencing and genomic instability by inhibiting the binding of RNA polymerase and transcription factors [86Singh AK, Bishayee A, Pandey AK. Targeting histone deacetylases with natural and synthetic agent: An emerging anticancer strategy. Nutrients 2018; 10(6): 731.
[http://dx.doi.org/10.3390/nu10060731] [PMID: 29882797] , 140Hendrich B, Bird A. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol Cell Biol 1998; 18(11): 6538-47.
[http://dx.doi.org/10.1128/MCB.18.11.6538] [PMID: 9774669] , 141Jones PA, Baylin SB. The epigenomics of cancer. Cell 2007; 128(4): 683-92.
[http://dx.doi.org/10.1016/j.cell.2007.01.029] [PMID: 17320506] ]. Hypermethylation is commonly observed at CpG islands in the promoter region of tumour suppressor genes in HCC. Suppressor of cytokine signalling, which regulates the JAK/STAT signalling pathway, was found to be silenced in more than 60% of HCC patients due to promoter hypermethylation [142Yoshikawa H, Matsubara K, Qian GS, et al. SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nat Genet 2001; 28(1): 29-35.
[http://dx.doi.org/10.1038/ng0501-29] [PMID: 11326271] ]. It has been reported that multiple tumor-related genes, such as the APC, E-Cadherin and Hypermethylated-In-Cancer (HIC)-18 genes, are silenced by DNA hypermethylation in HCC [143Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S. Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci USA 1995; 92(16): 7416-9.
[http://dx.doi.org/10.1073/pnas.92.16.7416] [PMID: 7543680] , 144Kanai Y, Hui AM, Sun L, et al. DNA hypermethylation at the D17S5 locus and reduced HIC-1 mRNA expression are associated with hepatocarcinogenesis. Hepatology 1999; 29(3): 703-9.
[http://dx.doi.org/10.1002/hep.510290338] [PMID: 10051471] ]. Stepwise increase in methylation of several genes was observed with the cancer progression. Upregulation of oncogenic signalling pathways such as JAK/STAT, Ras, and β-catenin/Wnt takes place by silencing tumour suppressors epigenetically in HCC has been revealed in genome-wide DNA methylation analysis [145Calvisi DF, Ladu S, Gorden A, et al. Mechanistic and prognostic significance of aberrant methylation in the molecular pathogenesis of human hepatocellular carcinoma. J Clin Invest 2007; 117(9): 2713-22.
[http://dx.doi.org/10.1172/JCI31457] [PMID: 17717605] ].
6. Drug targets in HCC
6.1. Multikinase Inhibitors
Sorafenib (BAY43-9006, Nexavar) is multikinase inhibitor with dual inhibitory activity against RAF/MEK/ERK (Raf-1, B-Raf) in the tumour cell and vascular growth factor inhibitor family (VEGFR1, VEGFR2) and platelets derived growth factor receptor (PDGFR, c-Kit) which promote tumour progression and angiogenesis. Therefore, sorafenib acts either directly on the tumour or on angiogenesis and inhibits tumour growth [146Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 2006; 5(10): 835-44.
[http://dx.doi.org/10.1038/nrd2130] [PMID: 17016424] ]. Sunitinib malate (SU11248, Sutent; Pfizer, NY, USA) and Linifanib (ABT-869) are also oral multikinase inhibitors that act on growth factors and receptor tyrosine kinases involved in angiogenesis and HCC progression [147Teramoto K, Ohshio Y, Fujita T, Hanaoka J, Kontani K. Simultaneous activation of T helper function can augment the potency of dendritic cell-based cancer immunotherapy. J Cancer Res Clin Oncol 2013; 139(5): 861-70.
[http://dx.doi.org/10.1007/s00432-013-1394-4] [PMID: 23411688] , 148Wörns MA, Schuchmann M, Düber C, Otto G, Galle PR, Weinmann A. Sunitinib in patients with advanced hepatocellular carcinoma after progression under sorafenib treatment. Oncology 2010; 79(1-2): 85-92.
[http://dx.doi.org/10.1159/000320363] [PMID: 21071995] ].
6.2. Inhibitors of Mesenchymal-Epithelial Transition factor (MET) Receptor
C-MET is a protein, encoded by MET oncogene, possesses tyrosine kinase activity involved in tumour development and metastasis [149Bottaro DP, Rubin JS, Faletto DL, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 1991; 251(4995): 802-4.
[http://dx.doi.org/10.1126/science.1846706] [PMID: 1846706] ]. Tivantinib (ARQ 197) and cabozantinib are MET inhibitors that act by binding to its dephosphorylated state which is responsible for inhibition of growth and apoptosis in human tumour cell line [150Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012; 12(2): 89-103.
[http://dx.doi.org/10.1038/nrc3205] [PMID: 22270953] , 151Fallahi P, Ferrari SM, Di Bari F, et al. Cabozantinib in thyroid cancer. Recent Patents Anticancer Drug Discov 2015; 10(3): 259-69.
[http://dx.doi.org/10.2174/1574892810666150708110816] [PMID: 26152149] ].
6.3. Angiogenesis Inhibitors
HCC is characterized by hyper vasculature resulting from higher expression of angiogenesis promoting factors viz., angiopoietin 2, PDGF, and VEGF [152Kumar S, Ahmad MK, Waseem M, Pandey AK. Drug targets for cancer treatment: An overview. Med Chem 2015; 5: 115-23. [https://doi.org/10.4172/2161-0444.1000252].]. Bevacizumab (Avastin; Genentech, CA, USA) is a humanised monoclonal antibody (mAb) acting on VEGF and one of the important drugs for colorectal cancer and liver metastasis of colorectal cancer [153Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350(23): 2335-42.
[http://dx.doi.org/10.1056/NEJMoa032691] [PMID: 15175435] ]. Brivanib (BMS-582664), an inhibitor of VEGF and FGF signalling has shown efficacy as a first-line treatment for advanced HCC patients [154Chou T, Finn RS. Brivanib: A review of development. Future Oncol 2012; 8(9): 1083-90.
[http://dx.doi.org/10.2217/fon.12.104] [PMID: 23030483] ]. Ramucirumab (Cyramza), a mAb, is an inhibitor of VEGFR-2 [155Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10(1): 25-34.
[http://dx.doi.org/10.1016/S1470-2045(08)70285-7] [PMID: 19095497] ].
6.4. PI3K/Akt/mTOR Inhibitors
Immunohistochemistry has shown that approximately 50% of HCC patients have activated mTOR pathway. This activation may be the result of increased signalling due to overexpression of ligands (EGF, IGF1, and IGF2) or may be due to mutant oncogenes (PI3KCA) or tumour suppressor genes (PTEN). Temsirolimus and Everolimus, an analogue of rapamycin are the inhibitors of mTOR [156Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology 2008; 48(4): 1312-27.
[http://dx.doi.org/10.1002/hep.22506] [PMID: 18821591] ].
CONCLUSION
HCC is common and aggressive malignant tumour worldwide with a dreadful outcome. Multiple factors including viruses, chemicals as well as inborn and acquired metabolic diseases are responsible for its development. HBV and HCV are the major risk factors for virus-induced HCC development through direct or indirect mechanisms. HBV DNA integration into the host genome induces genomic instability and eventually directs insertional mutagenesis. Epigenetic changes targeting the expression of tumour suppressor genes also occur early in the development of HCC. Since HCC is a complex disease, therefore it is difficult to characterize HCC with a single biomarker. Several diagnostic markers including α-fetoprotein, des-γ-carboxyprothrombin, glypican-3, golgi protein-73, squamous cell carcinoma antigen, miRNAs and altered DNA methylation pattern are associated with HCC. Thus, the investigation on a combination of biomarker might provide valuable insight for diagnosis and prognosis. Sever drug classes acting on various targets like multikinase inhibitors, MET receptor inhibitor, angiogenesis inhibitors and mTOR inhibitors have shown efficacy in the treatment of HCC patients. Further researches on HCC are necessary to identify new biomarkers and drugs for early diagnosis and effective treatment.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENT
AKS and RK acknowledge financial support from CSIR New Delhi in the form of Junior Research Fellowship. All the authors also acknowledge UGC-SAP and DST-FIST facilities of Biochemistry Department, University of Allahabad, Allahabad, India.
REFERENCES
| [1] | Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5): E359-86. [http://dx.doi.org/10.1002/ijc.29210] [PMID: 25220842] |
| [2] | Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol 2010; 7(8): 448-58. [http://dx.doi.org/10.1038/nrgastro.2010.100] [PMID: 20628345] |
| [3] | Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: Findings from the global burden of disease study 2013. Lancet 2016; 388(10049): 1081-8. [http://dx.doi.org/10.1016/S0140-6736(16)30579-7] [PMID: 27394647] |
| [4] | Grasso P. Experimental liver tumours in animals. In: Williams R, Johnson PJ, (eds.) Liver tumours. Bailliere’s clinical gastroenterology. 1987; London: Bailliere. pp. 183–305. |
| [5] | Bianchi L. Glucogen storage disease I and hepatocellular carcinoma. Eur J Pediatr 1993; 152(Sl):S63-70. [PMID: 8391447] |
| [6] | Alberts SR, Lanier AP, McMahon BJ, et al. Clustering of hepatocellular carcinoma in Alaska Native families. Genet Epidemiol 1991; 8(2): 127-39. [http://dx.doi.org/10.1002/gepi.1370080206] [PMID: 1655562] |
| [7] | Leong TYM, Leong ASY. Epidemiology and carcinogenesis of hepatocellular carcinoma. HPB 2005; 7(1): 5-15. [http://dx.doi.org/10.1080/13651820410024021] [PMID: 18333156] |
| [8] | Weinberg AG, Mize CE, Worthen HG. The occurrence of hepatoma in the chronic form of hereditary tyrosinemia. J Pediatr 1976; 88(3): 434-8. [http://dx.doi.org/10.1016/S0022-3476(76)80259-4] [PMID: 173827] |
| [9] | Kumar S, Pandey AK. Free Radicals: Health implications and their mitigation by herbals. Br J Med Med Res 2015; 7(6): 438-57. [http://dx.doi.org/10.9734/BJMMR/2015/16284] |
| [10] | Fregonese L, Stolk J. Hereditary alpha-1-antitrypsin deficiency and its clinical consequences. Orphanet J Rare Dis 2008; 3: 16. [http://dx.doi.org/10.1186/1750-1172-3-16] [PMID: 18565211] |
| [11] | Ganem D, Schneider RJ. Hepadnaviridae and their replication. In: Knipe, D.M., Howley, P.M., Griffin, D.E., Martin, M.A., Lamb, R.A., Roizman, B., et al., (eds.) 2001; Fields Virology. Vol. 4. Philadelphia, PA: Lippincott-Raven Publishers. |
| [12] | Hollinger FB, Liang TJ. Hepatitis B virus. In: Knipe DM, Howley PM, Griffin DE, Lamb R.A Martin MA, Roizman B, et al. (eds.), Fields Virology. Vol. 4. Philadelphia, PA: Lippincott-Raven Publishers. 2001; 2971-3036. |
| [13] | Gerlich WH, Robinson WS. Hepatitis B virus contains protein attached to the 5′ terminus of its complete DNA strand. Cell 1980; 21(3): 801-9. [http://dx.doi.org/10.1016/0092-8674(80)90443-2] [PMID: 7438207] |
| [14] | Report WHO. 2016.What is Hepatitis? |
| [15] | Klingmüller U, Schaller H. Hepadnavirus infection requires interaction between the viral pre-S domain and a specific hepatocellular receptor. J Virol 1993; 67(12): 7414-22. [PMID: 8230462] |
| [16] | Breiner KM, Urban S, Schaller H. Carboxypeptidase D (gp180), a Golgi-resident protein, functions in the attachment and entry of avian hepatitis B viruses. J Virol 1998; 72(10): 8098-104. [PMID: 9733850] |
| [17] | Kang HY, Lee S, Park SG, Yu J, Kim Y, Jung G. Phosphorylation of hepatitis B virus Cp at Ser87 facilitates core assembly. Biochem J 2006; 398(2): 311-7. [http://dx.doi.org/10.1042/BJ20060347] [PMID: 16740137] |
| [18] | Beck J, Nassal M. Hepatitis B virus replication. World J Gastroenterol 2007; 13(1): 48-64. [http://dx.doi.org/10.3748/wjg.v13.i1.48] [PMID: 17206754] |
| [19] | Jean-Jean O, Weimer T, de Recondo AM, Will H, Rossignol JM. Internal entry of ribosomes and ribosomal scanning involved in hepatitis B virus P gene expression. J Virol 1989; 63(12): 5451-4. [PMID: 2585611] |
| [20] | Alotaibi H, Atabey N, Diril N, Erdal E, Ozturk M. Molecular mechanism of hepatocellular carcinoma.Hepatocellular Carcinoma 2016; 43-63. [http://dx.doi.org/ 10.1007/978-3-319-34214-6_3] [http://dx.doi.org/10.1007/978-3-319-34214-6_3] |
| [21] | Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 2010; 176(1): 2-13. [http://dx.doi.org/10.2353/ajpath.2010.090675] [PMID: 20019184] |
| [22] | Sharma AK, Kumar S, Chashoo G, Saxena AK, Pandey AK. Cell cycle inhibitory activity of Piper longum against A549 cell line and its protective effect against metal-induced toxicity in rats. Ind J Biochem Biophys 2014; 51(5): 358-64. [PMID: 25630105] |
| [23] | Boylan JM, Gruppuso PA. D-type cyclins and G1 progression during liver development in the rat. Biochem Biophys Res Commun 2005; 330(3): 722-30. [http://dx.doi.org/10.1016/j.bbrc.2005.03.042] [PMID: 15809057] |
| [24] | Henley SA, Dick FA. The retinoblastoma family of proteins and their regulatory functions in the mammalian cell division cycle. Cell Div 2012; 7(1): 10. [http://dx.doi.org/10.1186/1747-1028-7-10] [PMID: 22417103] |
| [25] | Ortega S, Malumbres M, Barbacid M. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim Biophys Acta 2002; 1602(1): 73-87. [PMID: 11960696] |
| [26] | Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell 2008; 14(2): 159-69. [http://dx.doi.org/10.1016/j.devcel.2008.01.013] [PMID: 18267085] |
| [27] | Chen X, Cheung ST, So S, et al. Gene expression patterns in human liver cancers. Mol Biol Cell 2002; 13(6): 1929-39. [http://dx.doi.org/10.1091/mbc.02-02-0023] [PMID: 12058060] |
| [28] | Xu XR, Huang J, Xu ZG, et al. Insight into hepatocellular carcinogenesis at transcriptome level by comparing gene expression profiles of hepatocellular carcinoma with those of corresponding noncancerous liver. Proc Natl Acad Sci USA 2001; 98(26): 15089-94. [http://dx.doi.org/10.1073/pnas.241522398] [PMID: 11752456] |
| [29] | Murakami Y, Saigo K, Takashima H, et al. Large scaled analysis of hepatitis B virus (HBV) DNA integration in HBV related hepatocellular carcinomas. Gut 2005; 54(8): 1162-8. [http://dx.doi.org/10.1136/gut.2004.054452] [PMID: 16009689] |
| [30] | Tannapfel A, Grund D, Katalinic A, et al. Decreased expression of p27 protein is associated with advanced tumor stage in hepatocellular carcinoma. Int J Cancer 2000; 89(4): 350-5. [http://dx.doi.org/10.1002/1097-0215(20000720)89:4<350::AID-IJC6>3.0.CO;2-3] [PMID: 10956409] |
| [31] | Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 1965; 37(3): 614-36. [http://dx.doi.org/10.1016/0014-4827(65)90211-9] [PMID: 14315085] |
| [32] | Paradis V, Youssef N, Dargère D, et al. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol 2001; 32(3): 327-32. [http://dx.doi.org/10.1053/hupa.2001.22747] [PMID: 11274643] |
| [33] | Higashitsuji H, Higashitsuji H, Itoh K, et al. The oncoprotein gankyrin binds to MDM2/HDM2, enhancing ubiquitylation and degradation of p53. Cancer Cell 2005; 8(1): 75-87. [http://dx.doi.org/10.1016/j.ccr.2005.06.006] [PMID: 16023600] |
| [34] | Azechi H, Nishida N, Fukuda Y, et al. Disruption of the p16/cyclin D1/retinoblastoma protein pathway in the majority of human hepatocellular carcinomas. Oncology 2001; 60(4): 346-54. [http://dx.doi.org/10.1159/000058531] [PMID: 11408803] |
| [35] | Llovet JM, Chen Y, Wurmbach E, et al. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology 2006; 131(6): 1758-67. [http://dx.doi.org/10.1053/j.gastro.2006.09.014] [PMID: 17087938] |
| [36] | Liu H, Luan F, Ju Y, et al. In vitro transfection of the hepatitis B virus PreS2 gene into the human hepatocarcinoma cell line HepG2 induces upregulation of human telomerase reverse transcriptase. Biochem Biophys Res Commun 2007; 355(2): 379-84. [http://dx.doi.org/10.1016/j.bbrc.2007.01.160] [PMID: 17307151] |
| [37] | Eguchi A, Wree A, Feldstein AE. Biomarkers of liver cell death. J Hepatol 2014; 60(5): 1063-74. [http://dx.doi.org/10.1016/j.jhep.2013.12.026] [PMID: 24412608] |
| [38] | Okano H, Shiraki K, Inoue H, et al. Cellular FLICE/caspase-8-inhibitory protein as a principal regulator of cell death and survival in human hepatocellular carcinoma. Lab Invest 2003; 83(7): 1033-43. [http://dx.doi.org/10.1097/01.LAB.0000079328.76631.28] [PMID: 12861043] |
| [39] | Fabregat I. Dysregulation of apoptosis in hepatocellular carcinoma cells. World J Gastroenterol 2009; 15(5): 513-20. [http://dx.doi.org/10.3748/wjg.15.513] [PMID: 19195051] |
| [40] | Ranjan K, Pathak C. FADD regulates NF-κB activation and promotes ubiquitination of cFLIPL to induce apoptosis. Sci Rep 2016; 6: 22787. [http://dx.doi.org/10.1038/srep22787] [PMID: 26972597] |
| [41] | Yang YA, Zhang GM, Feigenbaum L, Zhang YE. Smad3 reduces susceptibility to hepatocarcinoma by sensitizing hepatocytes to apoptosis through downregulation of Bcl-2. Cancer Cell 2006; 9(6): 445-57. [http://dx.doi.org/10.1016/j.ccr.2006.04.025] [PMID: 16766264] |
| [42] | Chen RH, Su YH, Chuang RL, Chang TY. Suppression of transforming growth factor-beta-induced apoptosis through a phosphatidylinositol 3-kinase/Akt-dependent pathway. Oncogene 1998; 17(15): 1959-68. [http://dx.doi.org/10.1038/sj.onc.1202111] [PMID: 9788439] |
| [43] | Dennis PA, Rifkin DB. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci USA 1991; 88(2): 580-4. [http://dx.doi.org/10.1073/pnas.88.2.580] [PMID: 1846448] |
| [44] | Yamada T, De Souza AT, Finkelstein S, Jirtle RL. Loss of the gene encoding mannose 6-phosphate/insulin-like growth factor II receptor is an early event in liver carcinogenesis. Proc Natl Acad Sci USA 1997; 94(19): 10351-5. [http://dx.doi.org/10.1073/pnas.94.19.10351] [PMID: 9294214] |
| [45] | Hu TH, Huang CC, Lin PR, et al. Expression and prognostic role of tumor suppressor gene PTEN/MMAC1/TEP1 in hepatocellular carcinoma. Cancer 2003; 97(8): 1929-40. [http://dx.doi.org/10.1002/cncr.11266] [PMID: 12673720] |
| [46] | Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol 1995; 13: 29-60. [http://dx.doi.org/10.1146/annurev.iy.13.040195.000333] [PMID: 7612225] |
| [47] | Ferrari C, Chisari FV. In: The liver biology and pathobiology. Arias, I.M., (ed.), Lippincott Williams & Wilkins, Philadelphia. 2001; pp. 763-82. |
| [48] | Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev 2008; 18(1): 19-26. [http://dx.doi.org/10.1016/j.gde.2008.01.020] [PMID: 18440219] |
| [49] | Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med 2008; 14(3): 109-19. [http://dx.doi.org/10.1016/j.molmed.2007.12.007] [PMID: 18261959] |
| [50] | Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007; 317(5834): 121-4. [http://dx.doi.org/10.1126/science.1140485] [PMID: 17615358] |
| [51] | Muriel P. NF-kappaB in liver diseases: A target for drug therapy. J Appl Toxicol 2009; 29(2): 91-100. [http://dx.doi.org/10.1002/jat.1393] [PMID: 18937212] |
| [52] | Xiao C, Ghosh S. NF-kappaB, an evolutionarily conserved mediator of immune and inflammatory responses. Adv Exp Med Biol 2005; 560: 41-5. [http://dx.doi.org/10.1007/0-387-24180-9_5] [PMID: 15932018] |
| [53] | Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999; 18(49): 6853-66. [http://dx.doi.org/10.1038/sj.onc.1203239] [PMID: 10602461] |
| [54] | Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell 2002; 109(Suppl.): S81-96. [http://dx.doi.org/10.1016/S0092-8674(02)00703-1] [PMID: 11983155] |
| [55] | Luedde T, Schwabe RF. NF-κB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2011; 8(2): 108-18. [http://dx.doi.org/10.1038/nrgastro.2010.213] [PMID: 21293511] |
| [56] | Mauad TH, van Nieuwkerk CM, Dingemans KP, et al. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol 1994; 145(5): 1237-45. [PMID: 7977654] |
| [57] | Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004; 431(7007): 461-6. [http://dx.doi.org/10.1038/nature02924] [PMID: 15329734] |
| [58] | Haybaeck J, Zeller N, Wolf MJ, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell 2009; 16(4): 295-308. [http://dx.doi.org/10.1016/j.ccr.2009.08.021] [PMID: 19800575] |
| [59] | Aravalli RN, Steer CJ, Cressman EN. Molecular mechanisms of hepatocellular carcinoma. Hepatology 2008; 48(6): 2047-63. [http://dx.doi.org/10.1002/hep.22580] [PMID: 19003900] |
| [60] | Meyerson M, Gabriel S, Getz G. Advances in understanding cancer genomes through second-generation sequencing. Nat Rev Genet 2010; 11(10): 685-96. [http://dx.doi.org/10.1038/nrg2841] [PMID: 20847746] |
| [61] | Cho W, Ziogas DE, Katsios C, Roukos DH. Emerging personalized oncology: sequencing and systems strategies. Future Oncol 2012; 8(6): 637-41. [http://dx.doi.org/10.2217/fon.12.44] [PMID: 22764759] |
| [62] | Budhu A, Ji J, Wang XW. The clinical potential of microRNAs. J Hematol Oncol 2010; 3: 37. [http://dx.doi.org/10.1186/1756-8722-3-37] [PMID: 20925959] |
| [63] | Kumar V, Kato N, Urabe Y, et al. Genome-wide association study identifies a susceptibility locus for HCV-induced hepatocellular carcinoma. Nat Genet 2011; 43(5): 455-8. [http://dx.doi.org/10.1038/ng.809] [PMID: 21499248] |
| [64] | You JS, Jones PA. Cancer genetics and epigenetics: Two sides of the same coin? Cancer Cell 2012; 22(1): 9-20. [http://dx.doi.org/10.1016/j.ccr.2012.06.008] [PMID: 22789535] |
| [65] | Ijichi M, Takayama T, Matsumura M, Shiratori Y, Omata M, Makuuchi M. alpha-Fetoprotein mRNA in the circulation as a predictor of postsurgical recurrence of hepatocellular carcinoma: A prospective study. Hepatology 2002; 35(4): 853-60. [http://dx.doi.org/10.1053/jhep.2002.32100] [PMID: 11915031] |
| [66] | Sund M, Kalluri R. Tumor stroma derived biomarkers in cancer. Cancer Metastasis Rev 2009; 28(1-2): 177-83. [http://dx.doi.org/10.1007/s10555-008-9175-2] [PMID: 19259624] |
| [67] | Tatarinov IuS. Detection of embryo-specific alpha-globulin in the blood serum of a patient with primary liver cancer. Vopr Med Khim 1964; 10: 90-1. [PMID: 14207501] |
| [68] | Mizejewski GJ. Alpha-fetoprotein structure and function: relevance to isoforms, epitopes, and conformational variants. Exp Biol Med (Maywood) 2001; 226(5): 377-408. [http://dx.doi.org/10.1177/153537020122600503] [PMID: 11393167] |
| [69] | Gitlin D, Perricelli A, Gitlin JD. The presence of serum alpha-fetoprotein in sharks and its synthesis by fetal gastrointestinal tract and liver. Comp Biochem Physiol B 1973; 46(2): 207-15. [http://dx.doi.org/10.1016/0305-0491(73)90311-8] [PMID: 4127981] |
| [70] | Debruyne EN, Delanghe JR. Diagnosing and monitoring hepatocellular carcinoma with alpha-fetoprotein: new aspects and applications. Clin Chim Acta 2008; 395(1-2): 19-26. [http://dx.doi.org/10.1016/j.cca.2008.05.010] [PMID: 18538135] |
| [71] | Chayvialle JA, Ganguli PC. Radioimmunoassay of alpha-fetoprotein in human plasma. Lancet 1973; 1(7816): 1355-7. [http://dx.doi.org/10.1016/S0140-6736(73)91676-0] [PMID: 4122743] |
| [72] | Waldmann TA, McIntire KR. The use of a radioimmunoassay for alpha-fetoprotein in the diagnosis of malignancy. Cancer 1974; 34(4)(Suppl.): 1510-5. [http://dx.doi.org/10.1002/1097-0142(197410)34:8+<1510::AID-CNCR2820340824>3.0.CO;2-Y] [PMID: 4138906] |
| [73] | Sato Y, Nakata K, Kato Y, et al. Early recognition of hepatocellular carcinoma based on altered profiles of alpha-fetoprotein. N Engl J Med 1993; 328(25): 1802-6. [http://dx.doi.org/10.1056/NEJM199306243282502] [PMID: 7684823] |
| [74] | Spangenberg HC, Thimme R, Blum HE. Serum markers of hepatocellular carcinoma. Semin Liver Dis 2006; 26(4): 385-90. [http://dx.doi.org/10.1055/s-2006-951606] [PMID: 17051452] |
| [75] | Naraki T, Kohno N, Saito H, et al. gamma-Carboxyglutamic acid content of hepatocellular carcinoma-associated des-gamma-carboxy prothrombin. Biochim Biophys Acta 2002; 1586(3): 287-98. [http://dx.doi.org/10.1016/S0925-4439(01)00107-7] [PMID: 11997080] |
| [76] | Volk ML, Hernandez JC, Su GL, Lok AS, Marrero JA. Risk factors for hepatocellular carcinoma may impair the performance of biomarkers: a comparison of AFP, DCP, and AFP-L3. Cancer Biomark 2007; 3(2): 79-87. [http://dx.doi.org/10.3233/CBM-2007-3202] [PMID: 17522429] |
| [77] | Filmus J. The contribution of in vivo manipulation of gene expression to the understanding of the function of glypicans. Glycoconj J 2002; 19(4-5): 319-23. [http://dx.doi.org/10.1023/A:1025312819804] [PMID: 12975611] |
| [78] | Filmus J, Capurro M. The role of glypican-3 in the regulation of body size and cancer. Cell Cycle 2008; 7(18): 2787-90. [http://dx.doi.org/10.4161/cc.7.18.6672] [PMID: 18787398] |
| [79] | Sung YK, Hwang SY, Park MK, et al. Glypican-3 is overexpressed in human hepatocellular carcinoma. Cancer Sci 2003; 94(3): 259-62. [http://dx.doi.org/10.1111/j.1349-7006.2003.tb01430.x] [PMID: 12824919] |
| [80] | Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res 2005; 65(14): 6245-54. [http://dx.doi.org/10.1158/0008-5472.CAN-04-4244] [PMID: 16024626] |
| [81] | Kladney RD, Cui X, Bulla GA, Brunt EM, Fimmel CJ. Expression of GP73, a resident Golgi membrane protein, in viral and nonviral liver disease. Hepatology 2002; 35(6): 1431-40. [http://dx.doi.org/10.1053/jhep.2002.32525] [PMID: 12029628] |
| [82] | Mao Y, Yang H, Xu H, et al. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut 2010; 59(12): 1687-93. [http://dx.doi.org/10.1136/gut.2010.214916] [PMID: 20876776] |
| [83] | Suminami Y, Kishi F, Sekiguchi K, Kato H. Squamous cell carcinoma antigen is a new member of the serine protease inhibitors. Biochem Biophys Res Commun 1991; 181(1): 51-8. [http://dx.doi.org/10.1016/S0006-291X(05)81380-4] [PMID: 1958219] |
| [84] | Giannelli G, Fransvea E, Trerotoli P, et al. Clinical validation of combined serological biomarkers for improved hepatocellular carcinoma diagnosis in 961 patients. Clin Chim Acta 2007; 383(1-2): 147-52. [http://dx.doi.org/10.1016/j.cca.2007.05.014] [PMID: 17582392] |
| [85] | Pozzan C, Cardin R, Piciocchi M, et al. Diagnostic and prognostic role of SCCA-IgM serum levels in hepatocellular carcinoma (HCC). J Gastroenterol Hepatol 2014; 29(8): 1637-44. [http://dx.doi.org/10.1111/jgh.12576] [PMID: 24635038] |
| [86] | Singh AK, Bishayee A, Pandey AK. Targeting histone deacetylases with natural and synthetic agent: An emerging anticancer strategy. Nutrients 2018; 10(6): 731. [http://dx.doi.org/10.3390/nu10060731] [PMID: 29882797] |
| [87] | Guan XY, Fang Y, Sham JST, et al. Recurrent chromosome alterations in hepatocellular carcinoma detected by comparative genomic hybridization. Genes Chromosomes Cancer 2000; 29(2): 110-6. [http://dx.doi.org/10.1002/1098-2264(2000)9999:9999<::AID-GCC1022>3.0.CO;2-V] [PMID: 10959090] |
| [88] | Chen L, Chan THM, Yuan YF, et al. CHD1L promotes hepatocellular carcinoma progression and metastasis in mice and is associated with these processes in human patients. J Clin Invest 2010; 120(4): 1178-91. [http://dx.doi.org/10.1172/JCI40665] [PMID: 20335658] |
| [89] | Kim TM, Yim SH, Shin SH, et al. Clinical implication of recurrent copy number alterations in hepatocellular carcinoma and putative oncogenes in recurrent gains on 1q. Int J Cancer 2008; 123(12): 2808-15. [http://dx.doi.org/10.1002/ijc.23901] [PMID: 18803288] |
| [90] | Nishimura T, Nishida N, Itoh T, et al. Discrete breakpoint mapping and shortest region of overlap of chromosome arm 1q gain and 1p loss in human hepatocellular carcinoma detected by semiquantitative microsatellite analysis. Genes Chromosomes Cancer 2005; 42(1): 34-43. [http://dx.doi.org/10.1002/gcc.20117] [PMID: 15495198] |
| [91] | Iwata N, Yamamoto H, Sasaki S, et al. Frequent hypermethylation of CpG islands and loss of expression of the 14-3-3 sigma gene in human hepatocellular carcinoma. Oncogene 2000; 19(46): 5298-302. [http://dx.doi.org/10.1038/sj.onc.1203898] [PMID: 11077447] |
| [92] | Fang W, Piao Z, Simon D, Sheu JC, Huang S. Mapping of a minimal deleted region in human hepatocellular carcinoma to 1p36.13-p36.23 and mutational analysis of the RIZ (PRDM2) gene localized to the region. Genes Chromosomes Cancer 2000; 28(3): 269-75. [http://dx.doi.org/10.1002/1098-2264(200007)28:3<269::AID-GCC4>3.0.CO;2-K] [PMID: 10862032] |
| [93] | Zhang YJ, Ahsan H, Chen Y, et al. High frequency of promoter hypermethylation of RASSF1A and p16 and its relationship to aflatoxin B1-DNA adduct levels in human hepatocellular carcinoma. Mol Carcinog 2002; 35(2): 85-92. [http://dx.doi.org/10.1002/mc.10076] [PMID: 12325038] |
| [94] | Miyoshi Y, Iwao K, Nagasawa Y, et al. Activation of the beta-catenin gene in primary hepatocellular carcinomas by somatic alterations involving exon 3. Cancer Res 1998; 58(12): 2524-7. [PMID: 9635572] |
| [95] | Zondervan PE, Wink J, Alers JC, et al. Molecular cytogenetic evaluation of virus-associated and non-viral hepatocellular carcinoma: analysis of 26 carcinomas and 12 concurrent dysplasias. J Pathol 2000; 192(2): 207-15. [http://dx.doi.org/10.1002/1096-9896(2000)9999:9999<::AID-PATH690>3.0.CO;2-#] [PMID: 11004697] |
| [96] | Chochi Y, Kawauchi S, Nakao M, et al. A copy number gain of the 6p arm is linked with advanced hepatocellular carcinoma: an array-based comparative genomic hybridization study. J Pathol 2009; 217(5): 677-84. [http://dx.doi.org/10.1002/path.2491] [PMID: 19097070] |
| [97] | Oka Y, Waterland RA, Killian JK, et al. M6P/IGF2R tumor suppressor gene mutated in hepatocellular carcinomas in Japan. Hepatology 2002; 35(5): 1153-63. [http://dx.doi.org/10.1053/jhep.2002.32669] [PMID: 11981765] |
| [98] | Santoni-Rugiu E, Jensen MR, Thorgeirsson SS. Disruption of the pRb/E2F pathway and inhibition of apoptosis are major oncogenic events in liver constitutively expressing c-myc and transforming growth factor alpha. Cancer Res 1998; 58(1): 123-34. [PMID: 9426068] |
| [99] | Okamoto H, Yasui K, Zhao C, Arii S, Inazawa J. PTK2 and EIF3S3 genes may be amplification targets at 8q23-q24 and are associated with large hepatocellular carcinomas. Hepatology 2003; 38(5): 1242-9. [http://dx.doi.org/10.1053/jhep.2003.50457] [PMID: 14578863] |
| [100] | Liu M, Chen L, Chan THM, et al. Serum and glucocorticoid kinase 3 at 8q13.1 promotes cell proliferation and survival in hepatocellular carcinoma. Hepatology 2012; 55(6): 1754-65. [http://dx.doi.org/10.1002/hep.25584] [PMID: 22262416] |
| [101] | Yuan BZ, Miller MJ, Keck CL, Zimonjic DB, Thorgeirsson SS, Popescu NC. Cloning, characterization, and chromosomal localization of a gene frequently deleted in human liver cancer (DLC-1) homologous to rat RhoGAP. Cancer Res 1998; 58(10): 2196-9. [PMID: 9605766] |
| [102] | Wang G, Huang CH, Zhao Y, et al. Genetic aberration in primary hepatocellular carcinoma: correlation between p53 gene mutation and loss-of-heterozygosity on chromosome 16q21-q23 and 9p21-p23. Cell Res 2000; 10(4): 311-23. [http://dx.doi.org/10.1038/sj.cr.7290058] [PMID: 11191353] |
| [103] | Nishida N, Fukuda Y, Komeda T, et al. Amplification and overexpression of the cyclin D1 gene in aggressive human hepatocellular carcinoma. Cancer Res 1994; 54(12): 3107-10. [PMID: 8205525] |
| [104] | Fujiwara Y, Hoon DS, Yamada T, et al. PTEN / MMAC1 mutation and frequent loss of heterozygosity identified in chromosome 10q in a subset of hepatocellular carcinomas. Jpn J Cancer Res 2000; 91(3): 287-92. [http://dx.doi.org/10.1111/j.1349-7006.2000.tb00943.x] [PMID: 10760687] |
| [105] | Tsujiuchi T, Sugata E, Masaoka T, et al. Expression and DNA methylation patterns of Tslc1 and Dal-1 genes in hepatocellular carcinomas induced by N-nitrosodiethylamine in rats. Cancer Sci 2007; 98(7): 943-8. [http://dx.doi.org/10.1111/j.1349-7006.2007.00480.x] [PMID: 17428255] |
| [106] | Kuroki T, Fujiwara Y, Nakamori S, Imaoka S, Kanematsu T, Nakamura Y. Evidence for the presence of two tumour-suppressor genes for hepatocellular carcinoma on chromosome 13q. Br J Cancer 1995; 72(2): 383-5. [http://dx.doi.org/10.1038/bjc.1995.342] [PMID: 7640222] |
| [107] | Yasui K, Arii S, Zhao C, et al. TFDP1, CUL4A, and CDC16 identified as targets for amplification at 13q34 in hepatocellular carcinomas. Hepatology 2002; 35(6): 1476-84. [http://dx.doi.org/10.1053/jhep.2002.33683] [PMID: 12029633] |
| [108] | Ko E, Kim SJ, Joh JW, Park CK, Park J, Kim DH. CpG island hypermethylation of SOCS-1 gene is inversely associated with HBV infection in hepatocellular carcinoma. Cancer Lett 2008; 271(2): 240-50. [http://dx.doi.org/10.1016/j.canlet.2008.06.009] [PMID: 18639978] |
| [109] | Zhao X, Li J, He Y, et al. A novel growth suppressor gene on chromosome 17p13.3 with a high frequency of mutation in human hepatocellular carcinoma. Cancer Res 2001; 61(20): 7383-7. [PMID: 11606366] |
| [110] | Ma NF, Hu L, Fung JM, et al. Isolation and characterization of a novel oncogene, amplified in liver cancer 1, within a commonly amplified region at 1q21 in hepatocellular carcinoma. Hepatology 2008; 47(2): 503-10. [http://dx.doi.org/10.1002/hep.22072] [PMID: 18023026] |
| [111] | Chen L, Hu L, Chan THM, et al. Chromodomain helicase/adenosine triphosphatase DNA binding protein 1-like (CHD1l) gene suppresses the nucleus-to-mitochondria translocation of nur77 to sustain hepatocellular carcinoma cell survival. Hepatology 2009; 50(1): 122-9. [http://dx.doi.org/10.1002/hep.22933] [PMID: 19441106] |
| [112] | Wang Y, Wu MC, Sham JS, Zhang W, Wu WQ, Guan XY. Prognostic significance of c-myc and AIB1 amplification in hepatocellular carcinoma. A broad survey using high-throughput tissue microarray. Cancer 2002; 95(11): 2346-52. [http://dx.doi.org/10.1002/cncr.10963] [PMID: 12436441] |
| [113] | Wong CM, Lee JM, Ching YP, Jin DY, Ng IO. Genetic and epigenetic alterations of DLC-1 gene in hepatocellular carcinoma. Cancer Res 2003; 63(22): 7646-51. [PMID: 14633684] |
| [114] | Zhou XL, Thorgeirsson SS, Popescu NC. Restoration of DLC-1 gene expression induces apoptosis and inhibits both cell growth and tumorigenicity in human hepatocellular carcinoma cells. Oncogene 2004; 23(6):1308-13. [https://doi.org/10.1038/sj.onc.1207246][PMID: 14647417 |
| [115] | Lawrie CH, Gal S, Dunlop HM, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol 2008; 141(5): 672-5. [http://dx.doi.org/10.1111/j.1365-2141.2008.07077.x] [PMID: 18318758] |
| [116] | Gramantieri L, Ferracin M, Fornari F, et al. Cyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res 2007; 67(13): 6092-9. [http://dx.doi.org/10.1158/0008-5472.CAN-06-4607] [PMID: 17616664] |
| [117] | Kumar S, Pandey AK. Oxidative stress-related microRNAs as diagnostic markers: A newer Insight in diagnostics. In: Maurya P, Chandra P. (eds.) Oxidative Stress: Diagnostic Methods and Applications in Medical Science. Springer, Singapore. 2017; 113-125. [http://dx.doi.org/10.1007/978-981-10-4711-4_6] |
| [118] | Gramantieri L, Fornari F, Ferracin M, et al. MicroRNA-221 targets Bmf in hepatocellular carcinoma and correlates with tumor multifocality. Clin Cancer Res 2009; 15(16): 5073-81. [http://dx.doi.org/10.1158/1078-0432.CCR-09-0092] [PMID: 19671867] |
| [119] | Xiong Y, Fang JH, Yun JP, et al. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology 2010; 51(3): 836-45. [PMID: 20041405] |
| [120] | Yau WL, Lam CSC, Ng L, et al. Over-expression of miR-106b promotes cell migration and metastasis in hepatocellular carcinoma by activating epithelial-mesenchymal transition process. PLoS One 2013; 8(3): e57882. [http://dx.doi.org/10.1371/journal.pone.0057882] [PMID: 23483935] |
| [121] | Wang R, Zhao N, Li S, et al. MicroRNA-195 suppresses angiogenesis and metastasis of hepatocellular carcinoma by inhibiting the expression of VEGF, VAV2, and CDC42. Hepatology 2013; 58(2): 642-53. [http://dx.doi.org/10.1002/hep.26373] [PMID: 23468064] |
| [122] | Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 2007; 133(2): 647-58. [http://dx.doi.org/10.1053/j.gastro.2007.05.022] [PMID: 17681183] |
| [123] | Zhou L, Yang ZX, Song WJ, et al. MicroRNA-21 regulates the migration and invasion of a stem-like population in hepatocellular carcinoma. Int J Oncol 2013; 43(2): 661-9. [http://dx.doi.org/10.3892/ijo.2013.1965] [PMID: 23708209] |
| [124] | El Tayebi HM, Omar K, Hegy S, et al. Repression of miR-17-5p with elevated expression of E2F-1 and c-MYC in non-metastatic hepatocellular carcinoma and enhancement of cell growth upon reversing this expression pattern. Biochem Biophys Res Commun 2013; 434(3): 421-7. [http://dx.doi.org/10.1016/j.bbrc.2013.04.003] [PMID: 23583198] |
| [125] | Ding J, Huang S, Wu S, et al. Gain of miR-151 on chromosome 8q24.3 facilitates tumour cell migration and spreading through downregulating RhoGDIA. Nat Cell Biol 2010; 12(4): 390-9. [http://dx.doi.org/10.1038/ncb2039] [PMID: 20305651] |
| [126] | Luedde T. MicroRNA-151 and its hosting gene FAK (focal adhesion kinase) regulate tumor cell migration and spreading of hepatocellular carcinoma. Hepatology 2010; 52(3): 1164-6. [http://dx.doi.org/10.1002/hep.23854] [PMID: 20812359] |
| [127] | Song K, Han C, Wu T. Epigenetic regulation of miR-122 expression by PPAR gamma/RXR alpha complex and HBx in hepatocellular carcinoma. Hepatology 2012; 56: 609a-10a. [https://doi.org/10.1002/hep.26514]. [PMID: 23703729]. |
| [128] | Xu H, He JH, Xiao ZD, et al. Liver-enriched transcription factors regulate microRNA-122 that targets CUTL1 during liver development. Hepatology 2010; 52(4): 1431-42. [http://dx.doi.org/10.1002/hep.23818] [PMID: 20842632] |
| [129] | Zhang X, Liu S, Hu T, Liu S, He Y, Sun S. Up-regulated microRNA-143 transcribed by nuclear factor kappa B enhances hepatocarcinoma metastasis by repressing fibronectin expression. Hepatology 2009; 50(2): 490-9. [http://dx.doi.org/10.1002/hep.23008] [PMID: 19472311] |
| [130] | Ying Q, Liang L, Guo W, et al. Hypoxia-inducible microRNA-210 augments the metastatic potential of tumor cells by targeting vacuole membrane protein 1 in hepatocellular carcinoma. Hepatology 2011; 54(6): 2064-75. [http://dx.doi.org/10.1002/hep.24614] [PMID: 22144109] |
| [131] | Ji J, Zhao L, Budhu A, et al. Let-7g targets collagen type I alpha2 and inhibits cell migration in hepatocellular carcinoma. J Hepatol 2010; 52(5): 690-7. [http://dx.doi.org/10.1016/j.jhep.2009.12.025] [PMID: 20338660] |
| [132] | Yang X, Liang L, Zhang XF, et al. MicroRNA-26a suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting interleukin-6-Stat3 pathway. Hepatology 2013; 58(1): 158-70. [http://dx.doi.org/10.1002/hep.26305] [PMID: 23389848] |
| [133] | Fornari F, Gramantieri L, Ferracin M, et al. MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene 2008; 27(43): 5651-61. [http://dx.doi.org/10.1038/onc.2008.178] [PMID: 18521080] |
| [134] | Datta J, Kutay H, Nasser MW, et al. Methylation mediated silencing of MicroRNA-1 gene and its role in hepatocellular carcinogenesis. Cancer Res 2008; 68(13): 5049-58. [http://dx.doi.org/10.1158/0008-5472.CAN-07-6655] [PMID: 18593903] |
| [135] | Ding J, Huang S, Wang Y, et al. Genome-wide screening reveals that miR-195 targets the TNF-α/NF-κB pathway by down-regulating IκB kinase alpha and TAB3 in hepatocellular carcinoma. Hepatology 2013; 58(2): 654-66. [http://dx.doi.org/10.1002/hep.26378] [PMID: 23487264] |
| [136] | Law PT, Ching AK, Chan AW, et al. MiR-145 modulates multiple components of the insulin-like growth factor pathway in hepatocellular carcinoma. Carcinogenesis 2012; 33(6): 1134-41. [http://dx.doi.org/10.1093/carcin/bgs130] [PMID: 22431718] |
| [137] | Noh JH, Chang YG, Kim MG, et al. MiR-145 functions as a tumor suppressor by directly targeting histone deacetylase 2 in liver cancer. Cancer Lett 2013; 335(2): 455-62. [http://dx.doi.org/10.1016/j.canlet.2013.03.003] [PMID: 23499894] |
| [138] | Wang Y, Lee AT, Ma JZ, et al. Profiling microRNA expression in hepatocellular carcinoma reveals microRNA-224 up-regulation and apoptosis inhibitor-5 as a microRNA-224-specific target. J Biol Chem 2008; 283(19): 13205-15. [http://dx.doi.org/10.1074/jbc.M707629200] [PMID: 18319255] |
| [139] | Zhang Y, Takahashi S, Tasaka A, Yoshima T, Ochi H, Chayama K. Involvement of microRNA-224 in cell proliferation, migration, invasion, and anti-apoptosis in hepatocellular carcinoma. J Gastroenterol Hepatol 2013; 28(3): 565-75. [http://dx.doi.org/10.1111/j.1440-1746.2012.07271.x] [PMID: 22989374] |
| [140] | Hendrich B, Bird A. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol Cell Biol 1998; 18(11): 6538-47. [http://dx.doi.org/10.1128/MCB.18.11.6538] [PMID: 9774669] |
| [141] | Jones PA, Baylin SB. The epigenomics of cancer. Cell 2007; 128(4): 683-92. [http://dx.doi.org/10.1016/j.cell.2007.01.029] [PMID: 17320506] |
| [142] | Yoshikawa H, Matsubara K, Qian GS, et al. SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nat Genet 2001; 28(1): 29-35. [http://dx.doi.org/10.1038/ng0501-29] [PMID: 11326271] |
| [143] | Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S. Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci USA 1995; 92(16): 7416-9. [http://dx.doi.org/10.1073/pnas.92.16.7416] [PMID: 7543680] |
| [144] | Kanai Y, Hui AM, Sun L, et al. DNA hypermethylation at the D17S5 locus and reduced HIC-1 mRNA expression are associated with hepatocarcinogenesis. Hepatology 1999; 29(3): 703-9. [http://dx.doi.org/10.1002/hep.510290338] [PMID: 10051471] |
| [145] | Calvisi DF, Ladu S, Gorden A, et al. Mechanistic and prognostic significance of aberrant methylation in the molecular pathogenesis of human hepatocellular carcinoma. J Clin Invest 2007; 117(9): 2713-22. [http://dx.doi.org/10.1172/JCI31457] [PMID: 17717605] |
| [146] | Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 2006; 5(10): 835-44. [http://dx.doi.org/10.1038/nrd2130] [PMID: 17016424] |
| [147] | Teramoto K, Ohshio Y, Fujita T, Hanaoka J, Kontani K. Simultaneous activation of T helper function can augment the potency of dendritic cell-based cancer immunotherapy. J Cancer Res Clin Oncol 2013; 139(5): 861-70. [http://dx.doi.org/10.1007/s00432-013-1394-4] [PMID: 23411688] |
| [148] | Wörns MA, Schuchmann M, Düber C, Otto G, Galle PR, Weinmann A. Sunitinib in patients with advanced hepatocellular carcinoma after progression under sorafenib treatment. Oncology 2010; 79(1-2): 85-92. [http://dx.doi.org/10.1159/000320363] [PMID: 21071995] |
| [149] | Bottaro DP, Rubin JS, Faletto DL, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 1991; 251(4995): 802-4. [http://dx.doi.org/10.1126/science.1846706] [PMID: 1846706] |
| [150] | Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012; 12(2): 89-103. [http://dx.doi.org/10.1038/nrc3205] [PMID: 22270953] |
| [151] | Fallahi P, Ferrari SM, Di Bari F, et al. Cabozantinib in thyroid cancer. Recent Patents Anticancer Drug Discov 2015; 10(3): 259-69. [http://dx.doi.org/10.2174/1574892810666150708110816] [PMID: 26152149] |
| [152] | Kumar S, Ahmad MK, Waseem M, Pandey AK. Drug targets for cancer treatment: An overview. Med Chem 2015; 5: 115-23. [https://doi.org/10.4172/2161-0444.1000252]. |
| [153] | Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350(23): 2335-42. [http://dx.doi.org/10.1056/NEJMoa032691] [PMID: 15175435] |
| [154] | Chou T, Finn RS. Brivanib: A review of development. Future Oncol 2012; 8(9): 1083-90. [http://dx.doi.org/10.2217/fon.12.104] [PMID: 23030483] |
| [155] | Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10(1): 25-34. [http://dx.doi.org/10.1016/S1470-2045(08)70285-7] [PMID: 19095497] |
| [156] | Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology 2008; 48(4): 1312-27. [http://dx.doi.org/10.1002/hep.22506] [PMID: 18821591] |






