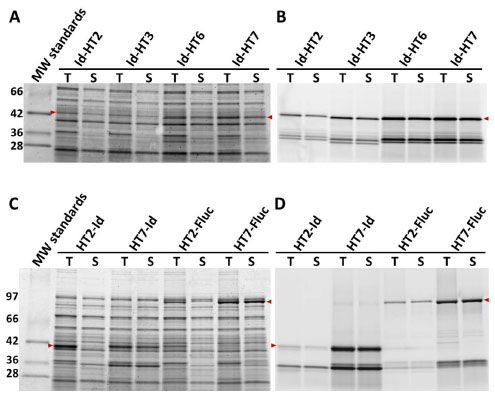
Fig. (9) Random mutagenesis of HT3 as a C-terminal tag resulted in further improved soluble and functional expression of fusions
to Id and Fluc. Id fusions (46 kDa) to HT, HT3, HT6 and HT7 were overexpressed in E. coli KRX at 30 °C and then lysate fractions containing
total (T) or soluble (S) protein were labeled to completion with the TMR-ligand and resolved by SDS-PAGE. Gels were imaged for
both total protein (SimplyBlue, panel A) and the amount of functional fusion (TMR fluorescence, panel B). Note the ~34 kDa band in panel
B which presumably represents truncation of the fusion. HT2 or HT7 fused to Id (46 kDa) or Luc (94 kDa) were overexpressed in E. coli
KRX at 30 °C and then lysate fractions containing total (T) or soluble (S) protein were labeled to completion with the TMR-ligand and resolved
by SDS-PAGE. Gels were imaged for both total protein (SimplyBlue, panel C) and the amount of functional fusion (TMR fluorescence,
panel D). Note the ~34 kDa band in panel B which presumably represents truncation of the fusion. Overlaid arrows indicate bands of
interest.