| Sr. No. | Substituent (R) | Time (min) | Yieldb ( %) | Melting point (ºC) |
||
|---|---|---|---|---|---|---|
| MWc | Cond | MWc | Cond | |||
| 6a | -CH3 | 4 | 30 | 96 | 90 | 230-232 |
| 6b | -CH(CH3)2 | 4 | 40 | 96 | 80 | 202-204 |
| 6c | -CH(CH3)CH2CH3 | 3 | 35 | 94 | 82 | 140-142 |
| 6d | -CH2C6H5 | 3 | 45 | 93 | 80 | 170-172 |
| 6e | -CH2CH2SCH3 | 3 | 45 | 94 | 82 | 156-158 |
| 6f | -CH2CH(CH3)2 | 4 | 35 | 94 | 84 | 233-235 |
| 6g | -CH2OH | 3 | 30 | 95 | 82 | 201-203 |
| 6h | -CH2SH | 4 | 35 | 94 | 84 | 206-208 |
| 6i | -CH2COOH | 4 | 50 | 96 | 80 | 182-184 |
| 6j | 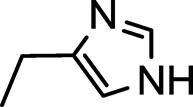 |
3 | 50 | 92 | 76 | 192-194 |
| 6k | -CH2C6H4OH | 3 | 45 | 92 | 72 | 150-152 |
| 6l | -CHOHCH3 | 3 | 35 | 94 | 78 | 170-172 |
a
Reaction condition (6a-l). Compound (4) (1 mmol), amino acids (5a-l) (1.2 mmol),
Method A: Microwave-assisted synthesis: potassium carbonate, ethanol, 70 ºC, 3-4 min.
Method B: Conventional synthesis: potassium carbonate, ethanol room temperature, 30-50 min.
b
Isolated yields.
c
Microwave-assisted.
d
Conventional condition.