- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Synthesis and Characterization of a Series of 1-Aryl-4-[Aryldiazenyl]-piperazines. Part II1. 1-Aryl-4-(2-Aryl-1-Diazenyl)-piperazines with Fluoro-, chloro-, Methyl-, Cyano- and Acetyl Substituents in The 1-Aryl Group
Karen O’Malleya, *, Keith Vaughanb, *
Abstract
This paper reports the synthesis and characterization of eight series of 1-aryl-4-(2-aryl-1-diazenyl)-piperazines (12 to 19). Several series of these triazenes have been synthesized by the diazotization of a primary arylamine followed by diazonium coupling with a secondary arylpiperazine . The arylpiperazines used in this study are: 1-phenylpiperazine, 1-(4-fluorophenyl-)piperazine, 1-(4-chlorophenyl-)piperazine, 1-(3,4-dichlorophenyl-)piperazine, 1-(2-methylphenyl-)-piperazine, 1-(4-acetophenyl-)-piperazine, 1-(2-pyridyl-)piperazine and 2-cyanophenylpiperazine. These new triazenes (series 12-19) have been identified with a cocktail of contemporary spectroscopic techniques, notably infra-red and nuclear magnetic spectroscopy, supported by high resolution electron ionization mass spectrometry.
Article Information
Identifiers and Pagination:
Year: 2016Volume: 3
First Page: 42
Last Page: 55
Publisher Id: CHEM-3-42
DOI: 10.2174/1874842201603010042
Article History:
Received Date: 31/7/2015Revision Received Date: 9/3/2016
Acceptance Date: 15/4/2016
Electronic publication date: 31/05/2016
Collection year: 2016
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to these authors at the Department of Chemistry, Saint Mary’s University Halifax, Nova Scotia B3H 3C3; Phone: (902)-420-5650; Fax: (902)-496-8104; E-mail: keith.vaughan@smu.ca, karen.omalley@alsglobal.com1 For part I in this series please see the Open Org Chem J, 2015, 9, 35-42.
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 31-7-2015 |
Original Manuscript | Synthesis and Characterization of a Series of 1-Aryl-4-[Aryldiazenyl]-piperazines. Part II1. 1-Aryl-4-(2-Aryl-1-Diazenyl)-piperazines with Fluoro-, chloro-, Methyl-, Cyano- and Acetyl Substituents in The 1-Aryl Group | |
INTRODUCTION
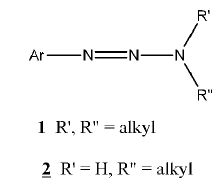
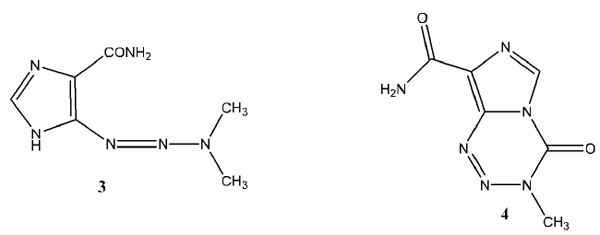
The chemistry of triazenes has been explored since the latter part of the eighteenth century. Most of the work centered on the chemistry of the 1-aryl-3,3-dialkyltriazenes (1) and to a lesser extent on the chemistry of the monoalkyltriazenes (2). The field of investigation of anti-tumour triazenes was established in the 1950s with the discovery of the biological activity of DTIC, also known as Dacarbazine, 5-(3,3-dimethyltriazen-1-yl)imidazole-4-carboxamide (3) [1Clarke, D.A.; Barclay, R.K.; Stock, C.C.; Rondestvedt, C.S. Jr. Triazenes as inhibitors of mouse sarcoma 180. Proc. Soc. Exp. Biol. Med., 1955, 90(2), 484-489.
[http://dx.doi.org/10.3181/00379727-90-22073] [PMID: 13273488] ] and in the 1980s with the discovery of temozolomide, also known as Temodal, (3,4-dihydro-3-methyl-4-oxoimidazo 5,1-d -as-tetrazine-8-carboxamide) (4) [2Arrowsmith, J.; Jennings, S.A.; Clark, A.S.; Stevens, M.F. Antitumor Imidazotetrazines. 40. Radiosyntheses of [4- C-Carbonyl]- and [3-N- C-Methyl]-8-carbamoyl-3-methylimidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (Temozolomide) for Positron Emission Tomography (PET) Studies. J. Med. Chem., 2002, 45, 5458-5870.
[http://dx.doi.org/10.1021/jm020936d] [PMID: 12459014] ]. The mechanism of action of these drugs is only partially understood, but researchers do agree that the effectiveness of triazene-based antitumour agents may be due to their ability to alkylate DNA. These drugs are still considered very effective therapies, especially Dacarbazine in the treatment of malignant melanomas [3Cocconi, G.; Bella, M.; Calabresi, F.; Tonato, M.; Canaletti, R.; Boni, C.; Buzzi, F.; Ceci, G.; Corgna, E.; Costa, P.; Lottici, R.; Papadia, F.; Sofra, M.C.; Bacchi, M. Treatment of metastatic malignant melanoma with dacarbazine plus tamoxifen. N. Engl. J. Med., 1992, 327(8), 516-523.
[http://dx.doi.org/10.1056/NEJM199208203270803] [PMID: 1635566] ] and Temodal for brain cancers [4Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; Curschmann, J.; Janzer, R.C.; Ludwin, S.K.; Gorlia, T.; Allgeier, A.; Lacombe, D.; Cairncross, J.G.; Eisenhauer, E.; Mirimanoff, R.O. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med., 2005, 352(10), 987-996.
[http://dx.doi.org/10.1056/NEJMoa043330] [PMID: 15758009] ].
From an alternative perspective, the medicinal chemistry of piperazine derivatives has attracted considerable interest. Research on arylpiperazines (5) is quite extensive due to their biological applications. They are especially known for their high affinity toward serotonin receptors, chiefly 5-HT1A receptors. Many common anxiolytics (antianxiety agents) and antidepressants incorporate arylpiperazines [5Caliendo, G.; Santagada, V.; Perissutti, E.; Fiorino, F. Derivatives as 5HT1A receptor ligands-past and present. Curr. Med. Chem., 2005, 12(15), 1721-1753.
[http://dx.doi.org/10.2174/0929867054367220] [PMID: 16029144] ]. It is believed that arylpiperazine derivatives act as 5-HT1A receptor antagonists [6Lopez-Rodriguez, M.L.; Ayala, D.; Benhamu, B.; Morcillo, M. J. Arylpiperazine derivatives acting as 5-HT1A receptors. Curr. Med. Chem., 2002, 9(4), 443-469.]. The possibility of combining the structural unit of a triazene with that of a piperazine raises interesting questions regarding the biological activity that might be generated from such a combination.
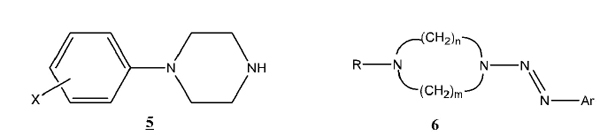
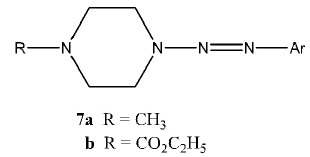
Vaughan et al. recently explored and expanded the range of triazene derivatives of piperazines in a series of papers. The structures investigated use (1, x)-diazocycloalkanes (6) where m = 1 or 2 and n = 2, 3, 4, or 5, as the general structure. The specific structures varied the R moiety, the homology of the piperazine ring, or a combination of the two. In a previous report [7Little, V.R.; Vaughan, K. Synthesis and Characterization of a series of 1-methyl-4-[2-aryl-1-diazenyl]piperazines and a series of ethyl 4-[2-aryl-1-diazenyl]-1-piperazinecarboxylates. Can. J. Chem., 2004, 82, 1294-1303.
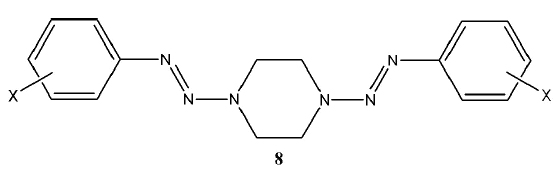
[http://dx.doi.org/10.1139/v04-081] ], the 1-(2-aryl-1-diazenyl-) 4-methylpiperazines (7a) were prepared by reaction of 1-methylpiperazine with the appropriate diazonium salt; this work also reported the analogous synthesis of the N-ethoxycarbonylpiperazines (7b). A subsequent study [8Little, V.R.; Tingley, R.; Vaughan, K. Triazene derivatives of [1,x]-diazacycloalkanes. Part III. Synthesis and characterization of a series of 1,4-di[2-aryl-1-diazenyl]piperazines. Can. J. Chem., 2005, 83, 471-476.
[http://dx.doi.org/10.1139/v05-064] ] of the diazonium coupling reaction with piperazine itself in 2 : 1 proportions resulted in the isolation and identification of the bis-triazene series (8). Some of the compounds in this series had been previously synthesized, but characterization had not been in depth [9Yanarates, E.; Disli, A. Yildirir. Y. New N,N’-bis-[substituted phenylazo]piperazines and their cleavage reactions in acetic acid. Org. Prep. Proced. Int., 1999, 31, 429-433.
[http://dx.doi.org/10.1080/00304949909355733] ].
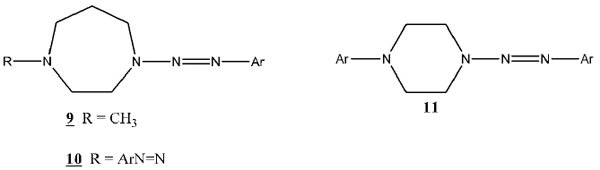
A further paper in Vaughan’s series [10Moser, S.L.; Vaughan, K. Synthesis and Characterization of a series of 4-methyl-1-2-aryl-1-diazenyl]-1,4-diazepanes and 1,4-di-[2-aryl-1-diazenyl]-1,4-diazepanes. Can. J. Chem., 2004, 82, 1725-1735.
[http://dx.doi.org/10.1139/v04-153] ] studied the effects of different piperazine ring homology, specifically diazepanes (9 and 10). One possible direction for further study arising from these papers would involve the synthesis of a series of compounds maintaining the (1, x)-diazocycloalkane base structure (6) with the piperazine ring, while further adding to different functional groups on the R group. The R group has included alkylated and esterified substituents, as well as the aryldiazenyl group in bis-triazenes (8 - 10). A logical next step would be to synthesize a series with the R group as an aryl moiety (11). This extension will further add to the information on similar series, and provide a synthetic method for future studies. Additional interest in these new compounds is derived from the potential medicinal applications of compounds of type 11.
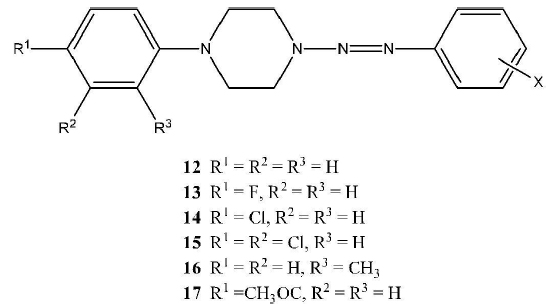
In this paper, several novel compounds of a new series of triazenes (12-17) have been synthesized and characterized.
The series has also been extended to the triazene series (18 and 19).
All compounds have been purified and characterized by IR and NMR spectroscopy and high resolution mass spectrometry (EI).
EXPERIMENTAL
For details of the experimental methods such as IR, NMR, mass spectrometry, melting point measurement, etc., see any of the prior references of this author [7Little, V.R.; Vaughan, K. Synthesis and Characterization of a series of 1-methyl-4-[2-aryl-1-diazenyl]piperazines and a series of ethyl 4-[2-aryl-1-diazenyl]-1-piperazinecarboxylates. Can. J. Chem., 2004, 82, 1294-1303.
[http://dx.doi.org/10.1139/v04-081] , 8Little, V.R.; Tingley, R.; Vaughan, K. Triazene derivatives of [1,x]-diazacycloalkanes. Part III. Synthesis and characterization of a series of 1,4-di[2-aryl-1-diazenyl]piperazines. Can. J. Chem., 2005, 83, 471-476.
[http://dx.doi.org/10.1139/v05-064] , 10Moser, S.L.; Vaughan, K. Synthesis and Characterization of a series of 4-methyl-1-2-aryl-1-diazenyl]-1,4-diazepanes and 1,4-di-[2-aryl-1-diazenyl]-1,4-diazepanes. Can. J. Chem., 2004, 82, 1725-1735.
[http://dx.doi.org/10.1139/v04-153] ].
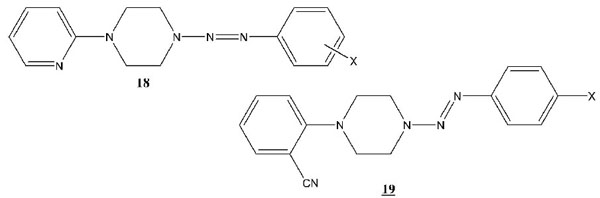
1-Aryl-4-(2-aryl-1-Diazenyl)-Piperazines (Series 12 to 19 Inclusive)
General Procedure
An aromatic primary amine (0.010 mol) was dissolved in 3M HCl (12.0 mL), and placed in an ice bath to cool to 0ºC. Sodium nitrite (0.011 mol), dissolved in water (3.0 mL) was added to the solution, and stirred for 0.5 hours. Concurrently, the appropriate aryl-piperazine (0.011 mol) was dissolved in water (1.0 mL), and cooled to 0ºC. If necessary to dissolve the alkylpiperazine, a small amount of 3M HCl (1.0 to 3.0 mL) was added. The piperazine solution was added slowly to the diazonium salt solution, and the resulting mixture was stirred for 0.5 hours. The solution was then neutralized with saturated sodium bicarbonate and left stirring in the cold for two hours. The product was collected using vacuum filtration if it was a solid and by extraction procedures if it was an oil. The solids were purified by recrystallization using an appropriate solvent. Oily products were isolated by extraction of the aqueous reaction mixture with dichloromethane, drying the organic layer over anhydrous magnesium sulphate, followed by evaporation of the solvent under vacuum.
RESULTS AND DISCUSSION
1-Aryl-4-(2-aryl-1-diazenyl)-piperazines: Synthesis
1-Aryl-4-(2-aryl-1-diazenyl)-piperazines (Series 12-17) were produced in good to excellent yields (20 - 99%) by reaction of a diazonium salt with a specific N-arylpiperazine. Physical data and IR spectroscopic data of these compounds are listed in Tables 1-6. The integrity of all compounds was verified by high-resolution mass spectrometry (EI) (see Tables 7-12). { See structures in the Introduction section above.}
The 1-(2-pyridyl)-4-(2-aryl-1-diazenyl-)piperazines (series 18) were prepared by diazotization of the arylamine and coupling with 1-(2-pyridyl-)piperazine. The 1-(2-cyanophenyl)-4-(2-aryl-1-diazenyl-)piperazines (series 19) were prepared by diazotization of the aryl amine and coupling with 1-(2-cyanophenyl-)piperazine. Physical data and infrared frequencies of the compounds of 18 and 19 are provided in Tables 13 and 15. Mass spectrometric data on these compounds are given in Tables 14 and 16.
Infrared Spectral Analysis
All compounds in series 12 - 19 were characterized by IR spectroscopy in order to confirm the presence of the appropriate aryl substituents and to confirm the expected substitution pattern of the aryl rings with reference to the OOP bending vibrations of aromatic-Hgroups C. All of the compounds in series 12 show out-of-plane (OOP) bending vibrations of the substituted benzene ring. The compounds of other series display analogous IR bands. Aliphatic and aromatic carbon-hydrogen stretches are not reported because the nujol peaks overwhelm the peaks of interest. In the case of the oil (12i), for which the IR spectrum was collected as a neat liquid, aliphatic carbon-hydrogen peaks were found at 2820 and 2976 cm-1, and an aromatic carbon-hydrogen peak was found at 3064 cm-1.
Significantly, it should be noted here that the analysis of the compounds of series 12 with the strongly electron-withdrawing substituent, p-nitro-, p-cyano- and p-methoxycarbonyl-, namely compounds 12h, 12a and 12b, is not included in this paper. The reason for this omission is that the products of these reactions are not pure and we believe that the formation of these triazenes is accompanied by the formation of isomeric azo-compounds. The formation of azo-compounds is only observed in the case of these three compounds. All other compounds of series 12 behave “normally” and afford single compounds characterized as the triazenes shown. Furthermore, the compounds of series 13 - 19 behave “normally“ and do not give rise to isomeric azo compounds. The evidence that corroborates the azo compound hypothesis is somewhat involved, requiring the synthesis and characterization of model azo compounds. These results will be described in full detail in a follow-up paper to this one.
1H NMR Spectral Analysis of Series 12 to 19
The proton nuclear magnetic resonance data of the members of series 12 to 19 is listed in Tables 17-24. These results provided unequivocal structural information for the new triazenes of series 12 to 19. Fig. (1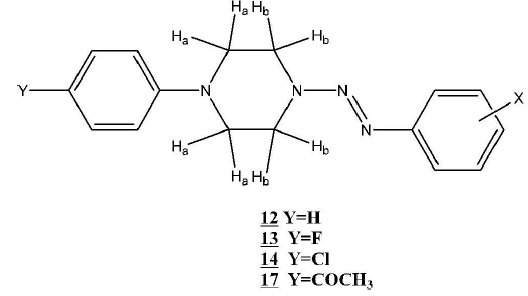 ) shows the labeling of the piperazine-ring protons described in the analysis information found in the Tables 17-24. The aromatic resonance signals of the para-disubstituted compounds (12c–g) displayed the expected multiplicity of an AA‘BB’ system.
) shows the labeling of the piperazine-ring protons described in the analysis information found in the Tables 17-24. The aromatic resonance signals of the para-disubstituted compounds (12c–g) displayed the expected multiplicity of an AA‘BB’ system.
 |
Fig. (1) Structure and substituents of 1-aryl-4-(2-aryl-1-diazenyl)-piperazines (12, 13,14, and 17)) with piperazine ring protons labeled. |
The piperazine resonance signals (12c - g and 12i) displayed the expected multiplicity of an A2B2 system. The 4-fluorophenyl ring resonance signals (13a - c) displayed a complicated splitting pattern. The signals were consistent within the series, and were found as a multiplet in the range δ6.96 - 7.04. This complicated pattern is due to the NMR activity of fluorine. The protons of the tolyl methyl substituent (13c) had δ2 a.39 singlet. The signal full NMR at information for series (13) can be found in Table 18.
The 1H NMR analysis information of the 1-(4-chlorophenyl)-4-(2-aryl-1-diazenyl)-piperazines (14) is shown in Table 19. 1H NMR spectral analysis of the 1-(3,4-dichlorophenyl)-4-(2-aryl-1-diazenyl)-piperazines (series 15) provided unequivocal structural information for these triazenes. The NMR analysis information can be found in Table 20, and the proton labeling is shown in Fig. (2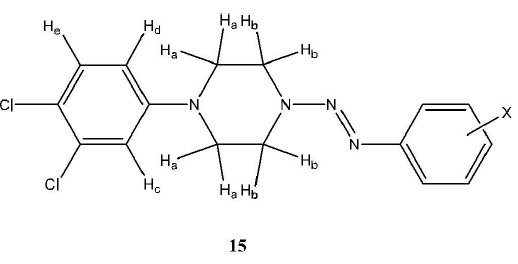 ).
).
The 3, 4-dichlorophenyl ring resonance signals (15a – h) displayed the general splitting pattern of a 1,3,4-trisubstituted aryl ring. Three hydrogen atoms, Hc, Hd, and He, were all identifiable (refer to Fig. 2 ). Proton assignments were based on multiplicity and coupling constants. Hc was assigned its position because of its doublet and small coupling constant due to W-coupling with Hd. Its doublet signals resonate in the range δ7.30 – 7.33. Hd was assigned its position because of its doublet of doublets and two coupling constants. The small coupling constant matches Hc’s, and its large coupling constant corresponds to vicinal coupling with He. Its doublet of doublet signals resonates in the range δ 6.78 – 6.81. He was assigned its position because of its doublet and large coupling constant which matches Hd. Its doublet signals resonate between δ 7.01 – 7.03.
). Proton assignments were based on multiplicity and coupling constants. Hc was assigned its position because of its doublet and small coupling constant due to W-coupling with Hd. Its doublet signals resonate in the range δ7.30 – 7.33. Hd was assigned its position because of its doublet of doublets and two coupling constants. The small coupling constant matches Hc’s, and its large coupling constant corresponds to vicinal coupling with He. Its doublet of doublet signals resonates in the range δ 6.78 – 6.81. He was assigned its position because of its doublet and large coupling constant which matches Hd. Its doublet signals resonate between δ 7.01 – 7.03.
The piperazine resonance signals (15a – h) also displayed the expected multiplicity of an A2B2 system. The triplet signals were found between δ 3.33 – 4.07. The O-methyl group of the alkoxy substituent (15d) appears as a singlet atδ 3.82. The methyl protons that were part of the acetyl group (15b) appear as a singlet at δ 2.60. The tolyl methyl substituent (15f) had a singlet signal at δ 2.39.
Table 21 shows the details of the 1H NMR analysis of the 1-(ortho-tolyl)-4-(2-aryl-1-diazenyl)-piperazines 16a-f. The piperazine resonance signals (16a - g) displayed the multiplicity of an A2B2 system. The triplet signals were found between δ3.08 – 4.46. 1H NMR spectral analysis of the 1-(4-Acetophenyl)-4-(2-aryl-1-diazenyl)-piperazines (17 a-c) (Fig. 1 ) gave very similar results which are detailed in Table 22.
) gave very similar results which are detailed in Table 22.
 |
Fig. (2) Structure and substituents of the 1-(3, 4-dichlorophenyl)-4-(2-aryl-1-diazenyl)-piperazines (15). |
1H NMR analysis of the 1-(2-pyridyl)-4-(2-aryl-1-diazenyl)-piperazines (18 a to h) provided unequivocal structural information for these triazenes. The NMR analysis information can be found in Table 23. 1H NMR analysis of the 1-(2-cyanophenyl)-4-(2-aryl-1-diazenyl)-piperazines (19 a to d) provided unequivocal structural information for these triazenes. The NMR analysis information can be found in Table 24.
CONCLUSION
Eight series of 1-aryl-4-(2-aryl-1-diazenyl)-piperazines were successfully synthesized: 1-phenyl-4-(2-aryl-1-diazenyl)-piperazines, 1-(4-fluorophenyl)-4-(2-aryl-1-diazenyl)-piperazines, 1-(4-chlorophenyl)-4-(2-aryl-1-diazenyl)-piperazines, 1-(3,4-dichlorophenyl)-4-(2-aryl-1-diazenyl)-piperazines, 1-(ortho-tolyl)-4-(2-aryl-1-diazenyl)-piperazines, 1-(4-acetophenyl)-4-(2-aryl-1-diazenyl)-piperazines, 1-(2-pyridyl)-4-(2-aryl-1-diazenyl)-piperazines and 1-(2-cyano phenyl)-4-(2-aryl-1-diazenyl)-piperazines. These compounds were identified by IR and 1H NMR, and confirmed by high-resolution mass spectroscopy (EI).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors are grateful to the Natural Sciences and Engineering Research Council of Canada (NSERC) for a Discovery Grant to the principal author (KV) and an Undergraduate Summer Research Award to Karen O’Malley (née Schurman). We are also grateful to the Faculty of Graduate Studies and Research at Saint Mary’s University for on-going support. We are also grateful to the Atlantic Region Magnetic Resonance Centre at Dalhousie University for providing NMR spectra, and to Dalhousie University for providing mass spectral data. In particular, we would like to thank Dr. Mike Lumsden for assistance with the NMR spectral data, and Mr. Xiao Feng for assistance with mass spectra.
REFERENCES
| [1] | Clarke, D.A.; Barclay, R.K.; Stock, C.C.; Rondestvedt, C.S. Jr. Triazenes as inhibitors of mouse sarcoma 180. Proc. Soc. Exp. Biol. Med., 1955, 90(2), 484-489. [http://dx.doi.org/10.3181/00379727-90-22073] [PMID: 13273488] |
| [2] | Arrowsmith, J.; Jennings, S.A.; Clark, A.S.; Stevens, M.F. Antitumor Imidazotetrazines. 40. Radiosyntheses of [4- C-Carbonyl]- and [3-N- C-Methyl]-8-carbamoyl-3-methylimidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (Temozolomide) for Positron Emission Tomography (PET) Studies. J. Med. Chem., 2002, 45, 5458-5870. [http://dx.doi.org/10.1021/jm020936d] [PMID: 12459014] |
| [3] | Cocconi, G.; Bella, M.; Calabresi, F.; Tonato, M.; Canaletti, R.; Boni, C.; Buzzi, F.; Ceci, G.; Corgna, E.; Costa, P.; Lottici, R.; Papadia, F.; Sofra, M.C.; Bacchi, M. Treatment of metastatic malignant melanoma with dacarbazine plus tamoxifen. N. Engl. J. Med., 1992, 327(8), 516-523. [http://dx.doi.org/10.1056/NEJM199208203270803] [PMID: 1635566] |
| [4] | Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; Curschmann, J.; Janzer, R.C.; Ludwin, S.K.; Gorlia, T.; Allgeier, A.; Lacombe, D.; Cairncross, J.G.; Eisenhauer, E.; Mirimanoff, R.O. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med., 2005, 352(10), 987-996. [http://dx.doi.org/10.1056/NEJMoa043330] [PMID: 15758009] |
| [5] | Caliendo, G.; Santagada, V.; Perissutti, E.; Fiorino, F. Derivatives as 5HT1A receptor ligands-past and present. Curr. Med. Chem., 2005, 12(15), 1721-1753. [http://dx.doi.org/10.2174/0929867054367220] [PMID: 16029144] |
| [6] | Lopez-Rodriguez, M.L.; Ayala, D.; Benhamu, B.; Morcillo, M. J. Arylpiperazine derivatives acting as 5-HT1A receptors. Curr. Med. Chem., 2002, 9(4), 443-469. |
| [7] | Little, V.R.; Vaughan, K. Synthesis and Characterization of a series of 1-methyl-4-[2-aryl-1-diazenyl]piperazines and a series of ethyl 4-[2-aryl-1-diazenyl]-1-piperazinecarboxylates. Can. J. Chem., 2004, 82, 1294-1303. [http://dx.doi.org/10.1139/v04-081] |
| [8] | Little, V.R.; Tingley, R.; Vaughan, K. Triazene derivatives of [1,x]-diazacycloalkanes. Part III. Synthesis and characterization of a series of 1,4-di[2-aryl-1-diazenyl]piperazines. Can. J. Chem., 2005, 83, 471-476. [http://dx.doi.org/10.1139/v05-064] |
| [9] | Yanarates, E.; Disli, A. Yildirir. Y. New N,N’-bis-[substituted phenylazo]piperazines and their cleavage reactions in acetic acid. Org. Prep. Proced. Int., 1999, 31, 429-433. [http://dx.doi.org/10.1080/00304949909355733] |
| [10] | Moser, S.L.; Vaughan, K. Synthesis and Characterization of a series of 4-methyl-1-2-aryl-1-diazenyl]-1,4-diazepanes and 1,4-di-[2-aryl-1-diazenyl]-1,4-diazepanes. Can. J. Chem., 2004, 82, 1725-1735. [http://dx.doi.org/10.1139/v04-153] |