- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Deformation Control and Mass Transfer in the Tunic of Halocynthia roretzi
Yoko Kato*
Abstract
Background:
It has been previously reported that the tunic of Halocynthia roretzi, mainly composed of cellulose, is actively deformed with mass transfer by the mechanical stimuli.
Objective:
In this study, how the tunic deforms in response to the mechanical environment was investigated.
Method:
The tunic specimen in the artificial seawater was still at 5˚C or underwent the mechanical stimuli at the temperature less than 10˚C. The mass and moisture content of the tunic, the concentrations of nitrate and dissolved organic matter in the artificial seawater used for the tunic, and the histological characteristics were evaluated.
Results:
The increase in mass of the tunic became lower as the region was closer to the bottom of Halocynthia roretzi. However, the decrease in mass caused by the mechanical stimuli was not different between the adjacent regions. Also, the tunic of the siphon, the tubular tissue for influx and efflux of the seawater, increased the mass more slowly after the stimuli. The size of the layer covering the outside of the tunic was inversely related to the increment in mass. The change in mass was corresponding to that in water content. The concentrations of nitrate and dissolved organic matter in the artificial seawater were enhanced 5 days after the stimuli while the concentration ratio of dissolved organic matter to nitrate was kept constant.
Conclusion:
The water content in the tunic was used for controlling the mass response to the mechanical environment.
Article Information
Identifiers and Pagination:
Year: 2018Volume: 5
First Page: 1
Last Page: 17
Publisher Id: CHEM-5-1
DOI: 10.2174/1874842201805010001
Article History:
Received Date: 30/12/2017Revision Received Date: 16/02/2018
Acceptance Date: 25/02/2018
Electronic publication date: 26/03/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Faculty of Engineering, Tohoku Gakuin University, 1-13-1, Chuo, Tagajo, Miyagi, 9858537, Japan, Tel: +81-22-368-7837; Fax: +81-22-368-7070; E-mail: ykato@mail.tohoku-gakuin.ac.jp
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 30-12-2017 |
Original Manuscript | Deformation Control and Mass Transfer in the Tunic of Halocynthia roretzi | |
1. INTRODUCTION
Cellulose, one of the most abundant substances in the world, can be found in various species, from higher plants to bacteria [1Wertz, J-L.; Bédué, O.; Mercider, J.P. Cellulose science and technology, 2010, ]. In Animalia (animal kingdom), the tunic of the ascidian, which covers the body of the ascidian entirely, is mainly composed of cellulose while the tunic shows various appearances from leathery to translucent one [2Berill, N.J. The tunicata, 1950, -4Das, S.M. On the structure and function of the ascidian test. J. Morphol., 1936, 59(3), 589-600.
[http://dx.doi.org/10.1002/jmor.1050590308] ]. The tunic surface is constantly replaced by new one with continuous secretion so that the thickness is kept constant [2Berill, N.J. The tunicata, 1950, ]. The tunic has blood vessels and various kinds of cells including hemocytes, receptor cells and neuro cells, which are dispersed in the tunic [2Berill, N.J. The tunicata, 1950, -4Das, S.M. On the structure and function of the ascidian test. J. Morphol., 1936, 59(3), 589-600.
[http://dx.doi.org/10.1002/jmor.1050590308] ]. In the defense system of Halocynthia roretzi, one of the solitary ascidians, the substances secreted from the hemocytes play an important role [5Yokosawa, H.; Sawada, H.; Abe, Y.; Numakunai, T.; Ishii, S. Galactose-specific lectin in the hemolymph of solitary ascidian, Halocynthia roretzi : isolation and characterization. Biochem. Biophys. Res. Commun., 1982, 107(2), 451-457.
[http://dx.doi.org/10.1016/0006-291X(82)91512-1] [PMID: 7126223] -12Azumi, K.; Satoh, N.; Yokosawa, H. Functional and structural characterization of hemocytes of the solitary ascidian. Halocynthia roretzi. J. Exp. Zool., 1993, 265, 309-316.
[http://dx.doi.org/10.1016/0145-305X(91)90042-W] ]. As these characteristics show, the tunic is not passive tissue, but dynamic tissue [2Berill, N.J. The tunicata, 1950, , 3Goodbody, I. The physiology of ascidians. Advances in Marine Biology., 1974, 12, 1-149.
[http://dx.doi.org/10.1016/S0065-2881(08)60457-5] ].
Halocynthia roretzi is entirely covered with its leathery tunic, where highly pure and crystalline cellulose Iβ [13Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc., 2002, 124(31), 9074-9082.
[http://dx.doi.org/10.1021/ja0257319] [PMID: 12149011] , 14Zhao, Y.; Li, J. Excellent chemical and material cellulose from tunicates: Diversity in cellulose production yield and chemical and morphological structures from different tunicate species. Cellulose, 2014, 21(5), 3427-3441.
[http://dx.doi.org/10.1007/s10570-014-0348-6] ], water-soluble chitin sulfate-like polysaccharide, which is predominantly composed of sulfated chitin [15Anno, K.; Otsuka, K.; Seno, N. A chitin sulfate-like polysaccharide from the test of the tunicate Halocynthia roretzi. Biochim. Biophys. Acta, 1974, 362(1), 215-219.
[http://dx.doi.org/10.1016/0304-4165(74)90043-9] [PMID: 4421550] , 16Wagner, G.P. Evolution and multi-functionality of the chitin system. EXS, 1994, 69, 559-577.
[http://dx.doi.org/10.1007/978-3-0348-7527-1_33] [PMID: 7994124] ], pseudokeratin [17Tuchiya, Y.; Suzuki, Y. Biochemical studies of the ascidian. Cynthia roretzi v. Drasche-VI. Nippon Suisan Gakkaishi, 1962, 28(2), 222-226.
[http://dx.doi.org/10.2331/suisan.28.222] ], α-smooth muscle actin [18Kato, Y. Active movement of the tunic in Halocynthia roretzi. Journal of Biomechanical Science and Engineering, 2010, 5(2), 163-174.
[http://dx.doi.org/10.1299/jbse.5.163] ], F-actin [19Kato, Y. The role of protein as a deformation controller in cellulose tissue. In Proceedings of ASME 2012 International Mechanical Engineering Congress and Exposition, Houston, Texas, USA, November 9-15, 2012; ASME: New York, USA, 2012; pp. 607-613
[http://dx.doi.org/10.1115/IMECE2012-89313] ], elastic fiber [18Kato, Y. Active movement of the tunic in Halocynthia roretzi. Journal of Biomechanical Science and Engineering, 2010, 5(2), 163-174.
[http://dx.doi.org/10.1299/jbse.5.163] ], nervous system [18Kato, Y. Active movement of the tunic in Halocynthia roretzi. Journal of Biomechanical Science and Engineering, 2010, 5(2), 163-174.
[http://dx.doi.org/10.1299/jbse.5.163] ], myocyte [18Kato, Y. Active movement of the tunic in Halocynthia roretzi. Journal of Biomechanical Science and Engineering, 2010, 5(2), 163-174.
[http://dx.doi.org/10.1299/jbse.5.163] ] and various types of hemocytes [12Azumi, K.; Satoh, N.; Yokosawa, H. Functional and structural characterization of hemocytes of the solitary ascidian. Halocynthia roretzi. J. Exp. Zool., 1993, 265, 309-316.
[http://dx.doi.org/10.1016/0145-305X(91)90042-W] ], have been found. While the mechanical stimuli on the tunic surface caused the contraction of the ascidian [4Das, S.M. On the structure and function of the ascidian test. J. Morphol., 1936, 59(3), 589-600.
[http://dx.doi.org/10.1002/jmor.1050590308] ], the tunic of Halocynthia roretzi itself, where other organs were removed, was also actively deformed by the administration of acetylcholine [18Kato, Y. Active movement of the tunic in Halocynthia roretzi. Journal of Biomechanical Science and Engineering, 2010, 5(2), 163-174.
[http://dx.doi.org/10.1299/jbse.5.163] ] and α-chymotrypsin [19Kato, Y. The role of protein as a deformation controller in cellulose tissue. In Proceedings of ASME 2012 International Mechanical Engineering Congress and Exposition, Houston, Texas, USA, November 9-15, 2012; ASME: New York, USA, 2012; pp. 607-613
[http://dx.doi.org/10.1115/IMECE2012-89313] ], mechanical [18Kato, Y. Active movement of the tunic in Halocynthia roretzi. Journal of Biomechanical Science and Engineering, 2010, 5(2), 163-174.
[http://dx.doi.org/10.1299/jbse.5.163] , 20Kato, Y. Mechanical senses and the tunic structure in Halocynthia roretzi. In Proceedings of The 2011 International Offshore and Polar Engineering Conference, Maui, Hawaii, USA, June 19-24, 2011; ISOPE: California, USA, 2011; pp.250-253.] and electrical [19Kato, Y. The role of protein as a deformation controller in cellulose tissue. In Proceedings of ASME 2012 International Mechanical Engineering Congress and Exposition, Houston, Texas, USA, November 9-15, 2012; ASME: New York, USA, 2012; pp. 607-613
[http://dx.doi.org/10.1115/IMECE2012-89313] ] stimuli at room temperature. As the metallo-protease secreted by the hemocyte, whose substrate was the same as chymotrypsin, behaved more active at 20˚C than 4˚C [10Azumi, K.; Yokosawa, H.; Ishii, S. Lipopolysaccharide induces release of a metallo-protease from hemocytes of the ascidian, Halocynthia roretzi. Dev. Comp. Immunol., 1991, 15(1-2), 1-7.
[http://dx.doi.org/10.1016/0145-305X(91)90041-V] [PMID: 2050243] ], the deformation reported previously [18Kato, Y. Active movement of the tunic in Halocynthia roretzi. Journal of Biomechanical Science and Engineering, 2010, 5(2), 163-174.
[http://dx.doi.org/10.1299/jbse.5.163] -20Kato, Y. Mechanical senses and the tunic structure in Halocynthia roretzi. In Proceedings of The 2011 International Offshore and Polar Engineering Conference, Maui, Hawaii, USA, June 19-24, 2011; ISOPE: California, USA, 2011; pp.250-253.] would occur when the protease was active. If the tunic itself senses and responds to the mechanical stimuli, the tissue structure of the tunic would be suitable for the mechanical environments. The deformation by the mechanical stimuli was associated with a change in mass of the tunic [19Kato, Y. The role of protein as a deformation controller in cellulose tissue. In Proceedings of ASME 2012 International Mechanical Engineering Congress and Exposition, Houston, Texas, USA, November 9-15, 2012; ASME: New York, USA, 2012; pp. 607-613
[http://dx.doi.org/10.1115/IMECE2012-89313] ], but a substance related to the change has not been identified. Considering that the tunic contains the water-soluble polysaccharide [15Anno, K.; Otsuka, K.; Seno, N. A chitin sulfate-like polysaccharide from the test of the tunicate Halocynthia roretzi. Biochim. Biophys. Acta, 1974, 362(1), 215-219.
[http://dx.doi.org/10.1016/0304-4165(74)90043-9] [PMID: 4421550] , 16Wagner, G.P. Evolution and multi-functionality of the chitin system. EXS, 1994, 69, 559-577.
[http://dx.doi.org/10.1007/978-3-0348-7527-1_33] [PMID: 7994124] ], influx and efflux of water could involve this change.
The ultraviolet absorbance in the spectroscopic analysis has been used to evaluate the components of the seawater such as bromide, nitrate and dissolved organic matter [21Armstrong, F.A.J.; Boalch, G.T. Volatile organic matter in algal culture media and sea water. Nature, 1960, 185, 761-762.
[http://dx.doi.org/10.1038/185761b0] -26Collos, Y.; Mornet, F.; Sciandra, A.; Waser, N.; Larson, A.; Harrison, P.J. An optical method for the rapid measurement of micromolar concentrations of nitrate in marine phytoplankton cultures. J. Appl. Phycol., 1999, 11, 179-184.
[http://dx.doi.org/10.1023/A:1008046023487] ]. The ultraviolet absorbance can be useful to evaluate substances discharged from the tunic when the tunic deforms.
In this study, how the tunic of Halocynthia roretzi deforms in response to the mechanical environment was investigated. Firstly, the response of the tunic to the mechanical environment with no mechanical stimulus was examined because the tunic could sense the mechanical environment and deform itself. The temperature was 5˚C in order to reduce the activity of the metallo-protease. Also, the mechanical stimuli were given to the tunic at the temperature lower than 10˚C. The mass and moisture content of the tunic specimen were measured in order to examine whether or not water is associated with a change in mass of the tunic. Substances discharged from the tunic were evaluated by spectroscopic analysis. Since the tissue structure could directly influence the deformation, the histological characteristics of the tunic were evaluated by observing the stained tissue with a light microscope.
2. MATERIALS AND METHODS
2.1. Tunic Specimens of Halocynthia roretzi and the Mechanical Environment with no Mechanical Stimulus
Figs. (1A and B
and B ) show one of the samples, Halocynthia roretzi, and the categories of the tunic specimens, respectively. The samples were obtained from Research Center for Marine Biology, Graduate School of Life Sciences, Tohoku University (Aomori, Japan) and Yamanaka Inc. (Miyagi, Japan). The tunic was removed from other organs with tweezers and trimming blades (Feather trimming blade; Feather Safety Razor, Co. Ltd., Osaka, Japan), and cut into appropriate sizes. The specimen was categorized into 4 groups as Figs. (1A
) show one of the samples, Halocynthia roretzi, and the categories of the tunic specimens, respectively. The samples were obtained from Research Center for Marine Biology, Graduate School of Life Sciences, Tohoku University (Aomori, Japan) and Yamanaka Inc. (Miyagi, Japan). The tunic was removed from other organs with tweezers and trimming blades (Feather trimming blade; Feather Safety Razor, Co. Ltd., Osaka, Japan), and cut into appropriate sizes. The specimen was categorized into 4 groups as Figs. (1A and B
and B ) show: Siphon, tubular parts for influx and efflux of the seawater; M1, regions with spines; M2, regions with no spine; and Bottom, regions contacting the bottom of the muscle. The artificial seawater was made by Reef Crystals (Aquarium Systems, Sarrebourg, France) and deionized water.
) show: Siphon, tubular parts for influx and efflux of the seawater; M1, regions with spines; M2, regions with no spine; and Bottom, regions contacting the bottom of the muscle. The artificial seawater was made by Reef Crystals (Aquarium Systems, Sarrebourg, France) and deionized water.
All the samples were put into the artificial seawater at 5˚C in the refrigerator (MRP-215FS; Panasonic Healthcare Co., Ltd., Tokyo, Japan) up to 10 days. Each day of the period is named as Day 1 and so on. The increment in the mass of the tunic (Δmr) and increment in the mass per day (Δmr/day) were evaluated as the section ‘Mass and Moisture Content of the Tunic’ shows. The number of the specimens in each group was as follows: Siphon, 22; M1, 27; M2, 15; Bottom, 25; Inside (inner regions of M2 and Bottom), 7; Outside (outer regions of M2 and Bottom), 6. The measurement frequency was as follows: Siphon, every day (n = 14), every day or two days (n = 6) and every day or three days (n=2); M1, every day (n = 19), every day or two days (n = 4), every day or three days (n = 3) and every day or six days (n = 1); M2, every day (n = 7); every day or two days (n = 4), every day or three days (n = 3) and every day or six days (n = 1); Bottom, every day (n = 19), every day or two days (n = 3), every day or three days (n = 3) and every day or six days (n = 1); Inside, every day (n = 7); Outside, every day (n = 6).
2.2. Mechanical Stimuli on the Tunic
The mechanical stimuli given to the tunic specimen at Day 1 were tapping the outer surface of the tunic specimen by tweezers for 3 minutes in the artificial seawater, at the temperature less than 10˚C, which was kept by ice packs. After the mechanical stimuli were given to the tunic specimen, the specimen and artificial seawater were at 5˚C in the refrigerator again, up to Day 7. The increment in the mass of the tunic (Δmr) and increment in the mass per day (Δmr/day) were evaluated as the section ‘Mass and Moisture Content of the Tunic’ shows. The number of the specimens was as follows: Siphon, 6; M1, 6; M2, 3; Bottom, 5. The frequency for measuring the mass was as follows: Siphon, every day (n = 4) and every day or five days (n = 2); M1, every day (n = 4) and every day or five days (n = 2); M2, every day (n = 2) and every day or five days (n = 1); Bottom, every day (n = 4) and every day or five days (n = 1).
2.3. Mass and Moisture Content of the Tunic
The mass of each specimen was measured by the balance (UW420S; Shimadzu Corporation, Kyoto, Japan) after removing the water at the surface of the specimen by wrapping it in paper wipers for 10 seconds (Kimwipe; Nippon Paper Crecia, Tokyo, Japan). The moisture content of the specimen was measured when the drying temperature was 200˚C by the moisture analyzer (MX-50; A&D, Tokyo, Japan) after removing the water at the sample surface by the aforementioned method and cutting the specimen into pieces by the trimming blade. The moisture content described by Equation (4) was measured when the change in the moisture content was less than 0.05%/min or 0.01%/min.
The mass of the specimen, m, was normalized by that just after making the specimen, mo, as follows:
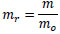 |
(1) |
where mr is the normalized mass. Hence, the increment in the mass of the tunic, Δmr, is described as follows:
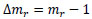 |
(2) |
The increment in the mass of the tunic per day, Δmr/day, is as follows:
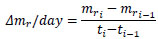 |
(3) |
where ti is the time for the ith measurement and mri is the normalized mass at ti. At giving the mechanical stimuli to the specimen, Δmr/day was calculated as follows, for convenience:
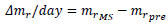 |
(3’) |
where mrMS and mrpre are the normalized mass at giving the mechanical stimuli and just before providing the stimuli at the same day, respectively. At the time of the measurement next to that at giving the stimuli, tpost, Δmr/day at tpost was calculated as follows:
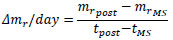 |
(3”) |
where mrpost is the mass at tpost, and tMS is the time at giving the stimuli.
The moisture content of the sample, C, is described as follows:
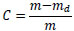 |
(4) |
where md is the mass of the dried specimen when the moisture content was measured. mrw, the mass of the water in the specimen normalized by mo, is described as follows:
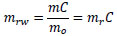 |
(5) |
Hence, the increment of mrw, Δmrw, is as follows:
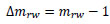 |
(6) |
The number of the specimens, which was used for evaluating Δmrw, was 35, including 4 specimens just after receiving the mechanical stimuli at Day 2 (Tapping for 6 min at the temperature less than 10˚C), and 2 specimens 5 days after receiving the aforementioned mechanical stimuli.
2.4. Substances Discharged from the Tunic
The absorbance of the artificial seawater used for the tunic specimen was measured by the spectrophotometer (UV-1280; Shimadzu Corporation, Kyoto, Japan). The concentrations of nitrate and dissolved organic matter in the artificial seawater were evaluated by the absorbance at 220 nm [26Collos, Y.; Mornet, F.; Sciandra, A.; Waser, N.; Larson, A.; Harrison, P.J. An optical method for the rapid measurement of micromolar concentrations of nitrate in marine phytoplankton cultures. J. Appl. Phycol., 1999, 11, 179-184.
[http://dx.doi.org/10.1023/A:1008046023487] ] and integrated absorbance from 250 - 350 nm [25Foster, P.; Morris, A.W. The use of ultra-violet absorption measurements for the estimation of organic pollution in inshore sea water. Water Res., 1971, 5, 19-27.
[http://dx.doi.org/10.1016/0043-1354(71)90059-5] ], respectively. The integrated absorbance, ΣA250-350, was equal to the average of absorbance at 250 – 350 nm, Ᾱ250-350, multiplied by the range of the wavelength, 100 nm. Hence, ΣA250-350 is described as follows:
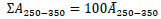 |
(7) |
The absorbance at 250 – 350 nm was measured every 0.5 nm. Every absorbance was measured three times at least and averaged. Also, the artificial seawater without usage was evaluated in the same way. The number of the artificial seawater sample was as follows: without usage, n = 1; for the tunic specimen without the mechanical stimuli, n = 11; just after the mechanical stimuli were given to the tunic specimen at Day 2 (Tapping for 6 min at the temperature less than 10˚C), n = 2; 5 days after the aforementioned mechanical stimuli were given, n = 2. The tunic specimen in the artificial seawater was less than 0.2 g/ml while that was 0.1 – 0.35 g/ml when the mechanical stimuli were given.
2.5. Histological Characteristics of the Tunic
The tissue structure of the tunic was evaluated by observing the stained specimen. The specimen was fixed in 20% formalin, which was diluted by the artificial seawater. After the fixation, the specimen was embedded in paraffin, sliced and stained by the following staining methods: Hematoxylin-Eosin stain, distribution of cells; Elastica-Masson stain, distribution of fibers; Periodic acid/Shiff reaction (PAS reaction), simple stain and Gram stain, distribution of bacteria. All the processes after the fixation were carried out by Surgical Pathology Japan, Inc. (Miyagi, Japan). The stained sample was observed by the light microscope (BX51; Olympus Corporation, Tokyo, Japan).
2.6. Statistical Analysis
The difference in average between two groups and that between the periods in the same group were assessed by two sample t-test and paired t-test, respectively. In addition, the coefficient of determination, R2, in the regression line was evaluated by F value which the following equation shows:
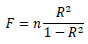 |
(8) |
where n is the number of samples. F shows the F-distribution, whose df1 and df2, degrees of freedom, is 1, and n-1, respectively. The level of significance was 0.05.
3. RESULTS
3.1. Tunic without the Mechanical Stimuli
Fig. (2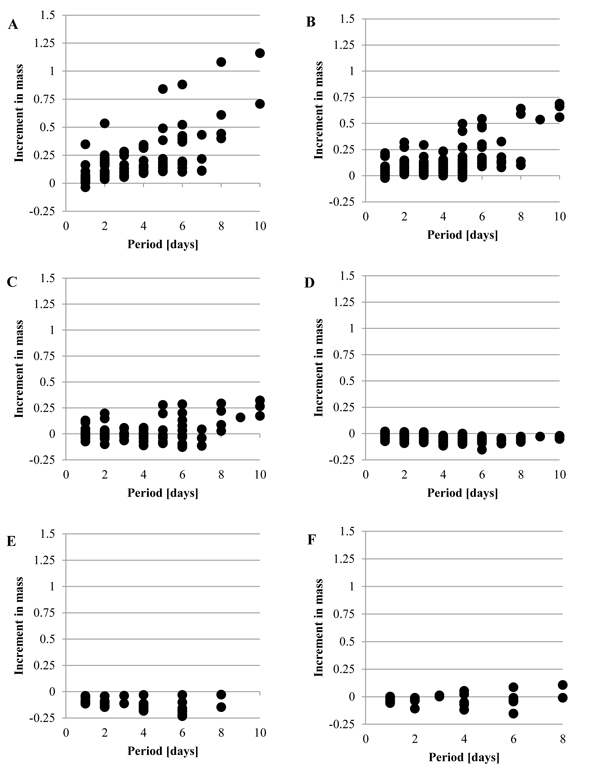 ) shows the increment in the mass, Δmr, when the tunic was put into the artificial seawater at 5˚C, without the mechanical stimuli. While Fig. (2A
) shows the increment in the mass, Δmr, when the tunic was put into the artificial seawater at 5˚C, without the mechanical stimuli. While Fig. (2A ) (Siphon) and Fig. (2B
) (Siphon) and Fig. (2B ) (M1) show that Δmr gradually increased as the period was longer, Fig. (2D
) (M1) show that Δmr gradually increased as the period was longer, Fig. (2D ) (Bottom) and Fig. (2E
) (Bottom) and Fig. (2E ) (Inside) show the decrease in the mass. Fig. (2C
) (Inside) show the decrease in the mass. Fig. (2C ) (M2) and Fig. (2F
) (M2) and Fig. (2F ) (Outside) show that Δmr increased as well as decreased. Fig. (3
) (Outside) show that Δmr increased as well as decreased. Fig. (3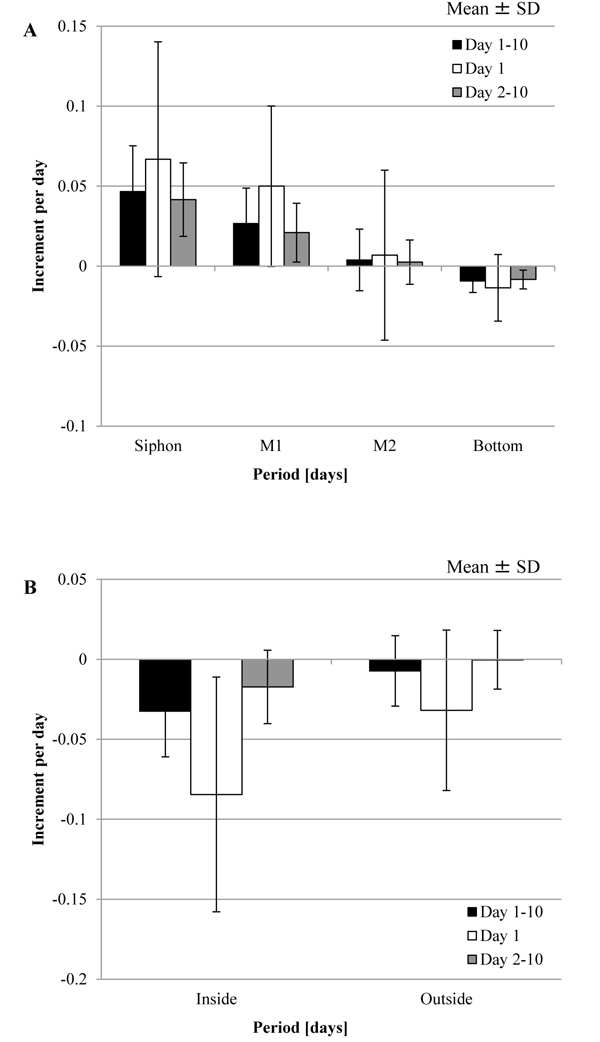 ) shows the increment per day, Δmr/day, in each group and division of the period, Day 1, Day 2-10 and Day 1-10, by mean ± SD (standard deviation). The period was divided into the three groups because the mechanical stimuli were given to the tunic at Day 1 in the other experiment. The result of the statistical analysis about Δmr/day is mentioned in Tables 1 and 2. As Fig. (3A
) shows the increment per day, Δmr/day, in each group and division of the period, Day 1, Day 2-10 and Day 1-10, by mean ± SD (standard deviation). The period was divided into the three groups because the mechanical stimuli were given to the tunic at Day 1 in the other experiment. The result of the statistical analysis about Δmr/day is mentioned in Tables 1 and 2. As Fig. (3A ), Fig. (3B
), Fig. (3B ) and Table 1 show, Δmr/day was significantly different between all the adjacent groups at Day 1-10: Siphon > M1; M1 > M2; M2 > Bottom; Outside > Inside. The adjacent groups except for the combination, Inside and Outside, were significantly different at Day 2-10 while the two combinations, M1 and M2, and, Inside and Outside, were at Day 1. According to these results, M1 > M2 was maintained through the period although other combinations were not. Fig. (3
) and Table 1 show, Δmr/day was significantly different between all the adjacent groups at Day 1-10: Siphon > M1; M1 > M2; M2 > Bottom; Outside > Inside. The adjacent groups except for the combination, Inside and Outside, were significantly different at Day 2-10 while the two combinations, M1 and M2, and, Inside and Outside, were at Day 1. According to these results, M1 > M2 was maintained through the period although other combinations were not. Fig. (3 ) and Table 2 mention that the absolute values of Δmr/day in M1 and Inside were significantly decreased from Day 1 to Day 2-10. If the increment in the mass is caused by decreasing the stress in the tunic, compression of M1 and stretch of Inside at the initial state (Day 0) would be larger than those on other regions.
) and Table 2 mention that the absolute values of Δmr/day in M1 and Inside were significantly decreased from Day 1 to Day 2-10. If the increment in the mass is caused by decreasing the stress in the tunic, compression of M1 and stretch of Inside at the initial state (Day 0) would be larger than those on other regions.
 |
Fig. (3) Increment in the mass of the tunic per day (Δmr/day) for the specimen without the mechanical stimuli. Δmr/day was evaluated at Day 1, Day 2–10, and Day 1–10 (mean ± standard deviation (SD)). A, Siphon (n = 22), M1 (n = 27), M2 (n = 15) and Bottom (n = 25); B, Inside (n = 7) and Outside (n = 6). The results of the statistical tests were described in Tables 1 and 2. |
3.2. Tunic with the Mechanical Stimuli
Fig. (4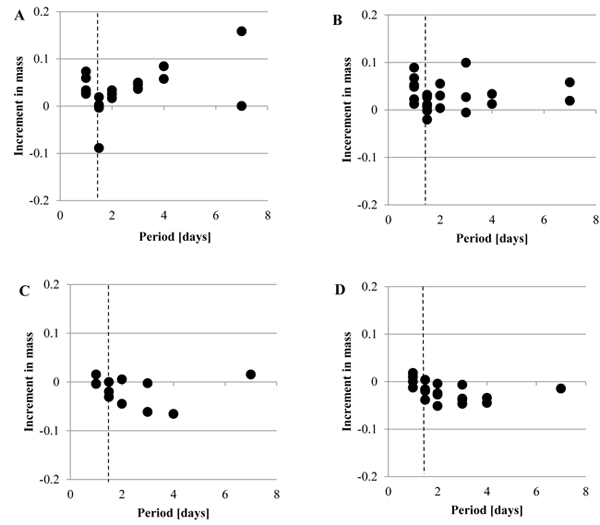 ) shows the increment in the mass, Δmr, when the mechanical stimuli were given to the tunic at Day 1. As Fig. (4A
) shows the increment in the mass, Δmr, when the mechanical stimuli were given to the tunic at Day 1. As Fig. (4A ) (Siphon), Fig. (4B
) (Siphon), Fig. (4B ) (M1), Fig. (4C
) (M1), Fig. (4C ) (M2) and Fig. (4D
) (M2) and Fig. (4D ) (Bottom) indicate, Δmr was decreased by the mechanical stimuli in each group while Δmr after the mechanical stimuli was diverse. The increment in the mass per day, Δmr/day, of each group is indicated by mean ± SD in Fig. (5
) (Bottom) indicate, Δmr was decreased by the mechanical stimuli in each group while Δmr after the mechanical stimuli was diverse. The increment in the mass per day, Δmr/day, of each group is indicated by mean ± SD in Fig. (5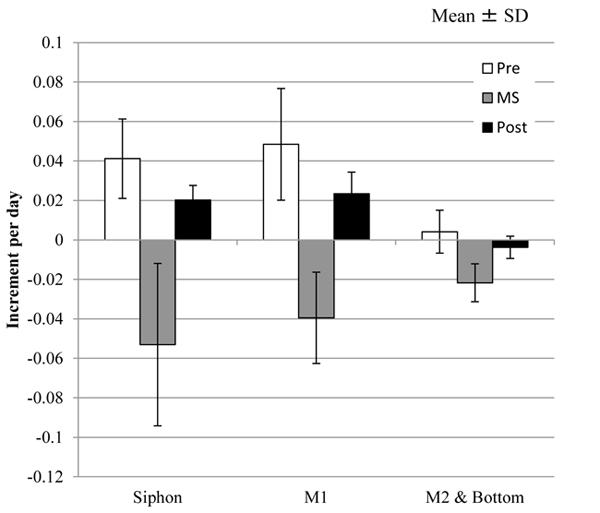 ), where the number of the samples in M2 was small (n = 3) so that the group named as M2 & Bottom, including the samples in both the groups, was organized. In addition, the period was divided into 3 groups: Pre, before giving the mechanical stimuli to the tunic specimen (corresponding to Day 1); MS, at giving the mechanical stimuli; Post, after giving the mechanical stimuli (corresponding to Day 2-10). Tables 1 and 2 show the results of the statistical analysis about Δmr/day. According to Fig. (5
), where the number of the samples in M2 was small (n = 3) so that the group named as M2 & Bottom, including the samples in both the groups, was organized. In addition, the period was divided into 3 groups: Pre, before giving the mechanical stimuli to the tunic specimen (corresponding to Day 1); MS, at giving the mechanical stimuli; Post, after giving the mechanical stimuli (corresponding to Day 2-10). Tables 1 and 2 show the results of the statistical analysis about Δmr/day. According to Fig. (5 ) and Table 1, Δmr/day in M1 was significantly larger than that in M2 & Bottom at Pre and Post while Δmr/day in Spine and M1 did not show significant difference in all the divisions of the period. At MS, there was no significant difference between all the adjacent groups. As Fig. (5
) and Table 1, Δmr/day in M1 was significantly larger than that in M2 & Bottom at Pre and Post while Δmr/day in Spine and M1 did not show significant difference in all the divisions of the period. At MS, there was no significant difference between all the adjacent groups. As Fig. (5 ) and Table 2 show, all the combinations of the period divisions in Siphon and M1 indicated the significant differences in Δmr/day: Pre > MS, Post > MS and Pre > Post. While Δmr/day in M2 & Bottom at MS was also significantly smaller than those of Pre and Post, the difference between Δmr/day in Pre and Post was not significant.
) and Table 2 show, all the combinations of the period divisions in Siphon and M1 indicated the significant differences in Δmr/day: Pre > MS, Post > MS and Pre > Post. While Δmr/day in M2 & Bottom at MS was also significantly smaller than those of Pre and Post, the difference between Δmr/day in Pre and Post was not significant.
 |
Fig. (5) Increment in the mass per day for the specimen with the mechanical stimuli (Δmr/day). Δmr/day was calculated in each group for the following three divisions of the period: Pre, before giving the mechanical stimuli to the specimen; MS, at giving the mechanical stimuli; Post, after giving the mechanical stimuli. The results of the statistical tests were described in Tables 1 and 2. Siphon, n = 6; M1, n = 6; M2 & Bottom, n = 8. |
Because that Pre and Post are corresponding to Day 1 and Day 2-10 in the tunic specimens without the mechanical stimuli, respectively, Δmr/day in these corresponding periods was compared. As Tables 1 and 2 show, the results of the statistical tests related to Δmr/day of Siphons were changed by the mechanical stimuli. As Table 1 shows, Δmr/day of Siphon and M1 were significantly different at Day 2-10, but those at Post were not. Also, Δmr/day of Siphon at Day 1 was not significantly different from that at Day 2-10, but Δmr/day at Pre was significantly larger than that at Post. according to Table 2. Fig. (6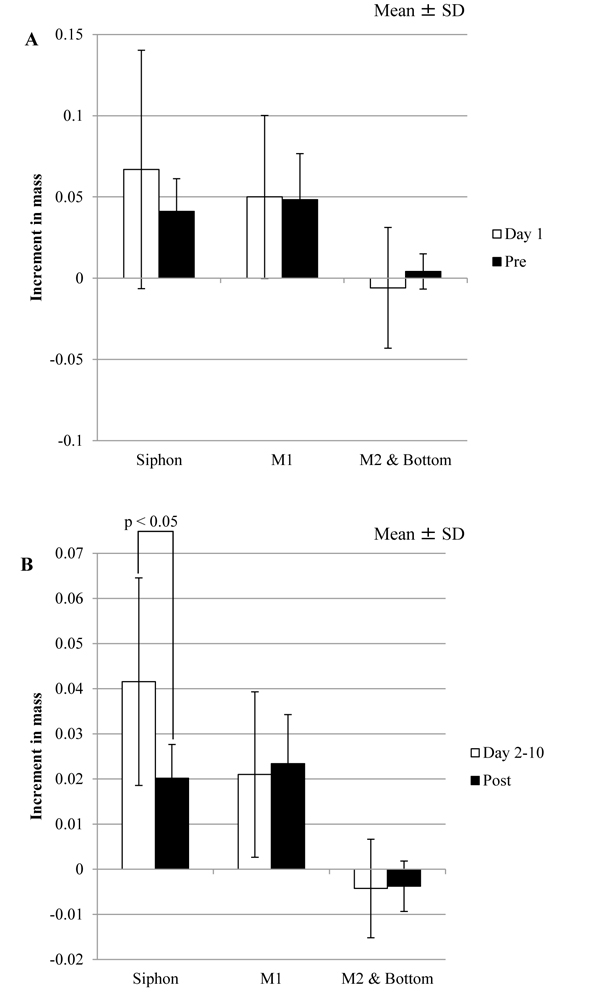 ) shows the comparison between the corresponding divisions of the period in the same regions. Δmr/day of Siphon at Post was significantly smaller than that at Day 2-10. These results indicate that the influence of the mechanical stimuli on Siphon would be larger than those on other regions.
) shows the comparison between the corresponding divisions of the period in the same regions. Δmr/day of Siphon at Post was significantly smaller than that at Day 2-10. These results indicate that the influence of the mechanical stimuli on Siphon would be larger than those on other regions.
3.3. Mass and Water in the Tunic
The increment in the mass of water at the specimen, Δmrw, and that in the mass of the specimen, Δmr, are shown in Fig. (7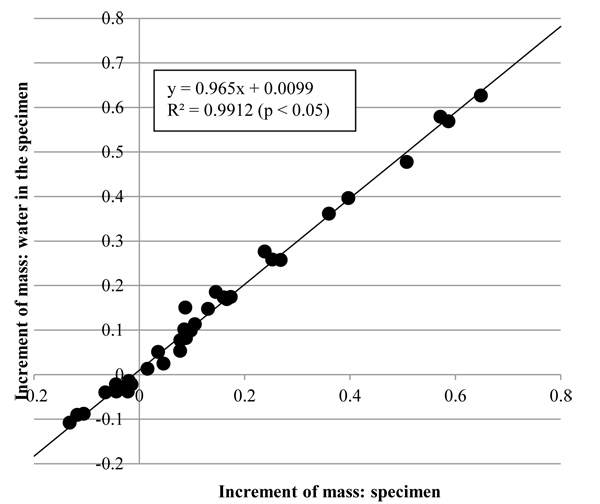 ). As the coefficient of determination, R2, of the regression line in Fig. (7
). As the coefficient of determination, R2, of the regression line in Fig. (7 ) show, Δmrw was significantly proportional to Δmr. Also, the gradient of the regression line was close to 1 so that Δmr would approximate to Δmrw. The results indicate that change in mass of the tunic would be caused by influx and efflux of water.
) show, Δmrw was significantly proportional to Δmr. Also, the gradient of the regression line was close to 1 so that Δmr would approximate to Δmrw. The results indicate that change in mass of the tunic would be caused by influx and efflux of water.
3.4. Substance Discharged from the Tunic
Fig. (8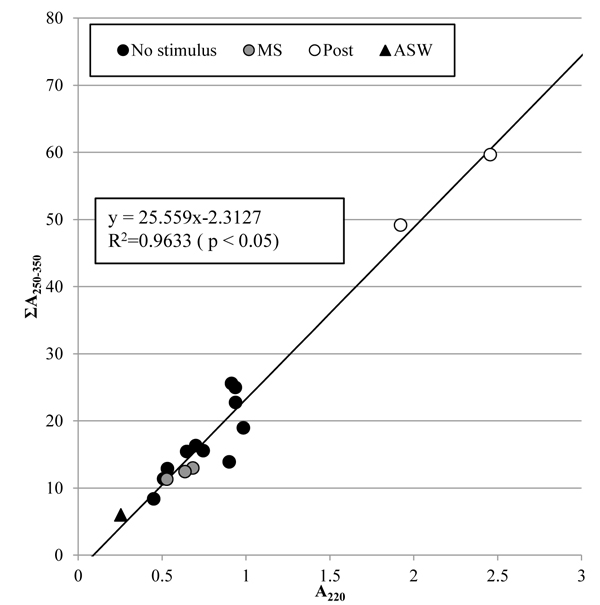 ) shows the concentrations of nitrate and dissolved organic matter in the artificial seawater, which are corresponding to the absorbance at 220 nm, A220, and the integrated absorbance from 250 nm to 350 nm, ΣA250-350, respectively. The coefficient of determination, R2, of the regression line for all the samples shows that the concentration of dissolved organic matter was significantly proportional to that of nitrate. Change in the concentrations of nitrate and dissolved organic matter was not clear just after the tunic was mechanically stimulated, but became clear 5 days after. This result indicates that the tunic would secrete the same substances whether or not the mechanical stimuli were given to it, but the mechanical stimuli could promote the secretion slowly.
) shows the concentrations of nitrate and dissolved organic matter in the artificial seawater, which are corresponding to the absorbance at 220 nm, A220, and the integrated absorbance from 250 nm to 350 nm, ΣA250-350, respectively. The coefficient of determination, R2, of the regression line for all the samples shows that the concentration of dissolved organic matter was significantly proportional to that of nitrate. Change in the concentrations of nitrate and dissolved organic matter was not clear just after the tunic was mechanically stimulated, but became clear 5 days after. This result indicates that the tunic would secrete the same substances whether or not the mechanical stimuli were given to it, but the mechanical stimuli could promote the secretion slowly.
3.5. Histological Characteristics of the Tunic
The distribution of bacteria in the tunic at Day 5 was examined by Hematoxylin-Eosin stain (Fig. (9A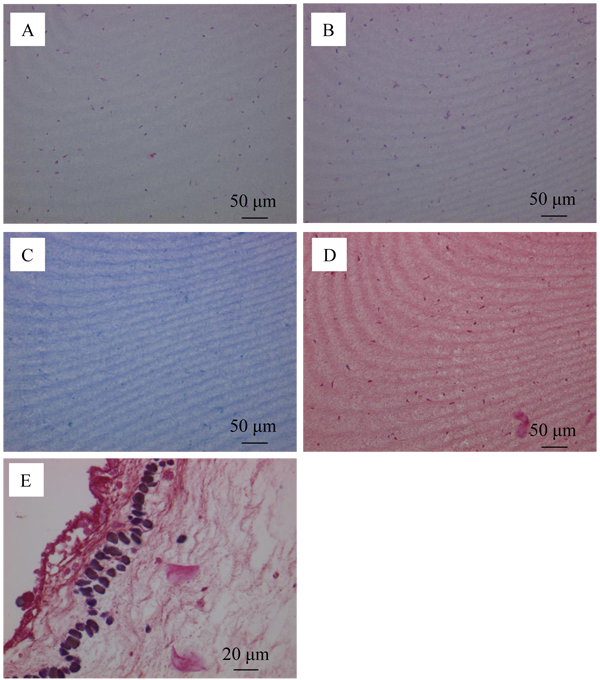 )), PAS reaction (Fig. (9B
)), PAS reaction (Fig. (9B )), simple stain (Fig. (9C
)), simple stain (Fig. (9C )) and Gram stain (Figs. (9D
)) and Gram stain (Figs. (9D and E
and E )). Fig. (9A
)). Fig. (9A ) shows that the tunic specimen had cells because cytoplasm (pink) and nuclei (purple) were observed. The distribution of the region positive for PAS reaction in Fig. (9B
) shows that the tunic specimen had cells because cytoplasm (pink) and nuclei (purple) were observed. The distribution of the region positive for PAS reaction in Fig. (9B ) is similar to that for cytoplasm and nuclei in Fig. (9A
) is similar to that for cytoplasm and nuclei in Fig. (9A ). Figs. (9C
). Figs. (9C and D
and D ) show that the region positive for simple stain and Gram stain was not observed. However, the positive regions, which would be corresponding to not bacteria, but phagocytes, were found in the area contacting the muscle as shown in Fig. (9E
) show that the region positive for simple stain and Gram stain was not observed. However, the positive regions, which would be corresponding to not bacteria, but phagocytes, were found in the area contacting the muscle as shown in Fig. (9E ). These characteristics were the same as those of the control group (Day 0) so that the propagation of bacteria would be prevented in the tunic specimen.
). These characteristics were the same as those of the control group (Day 0) so that the propagation of bacteria would be prevented in the tunic specimen.
 |
Fig. (7) Increments in the mass of the tunic (Δmr) and mass of water in the tunic (Δmrw). Δmr was significantly proportional to Δmrw (n = 35, p < 0.05). R2, coefficient of determination. |
Fig. (10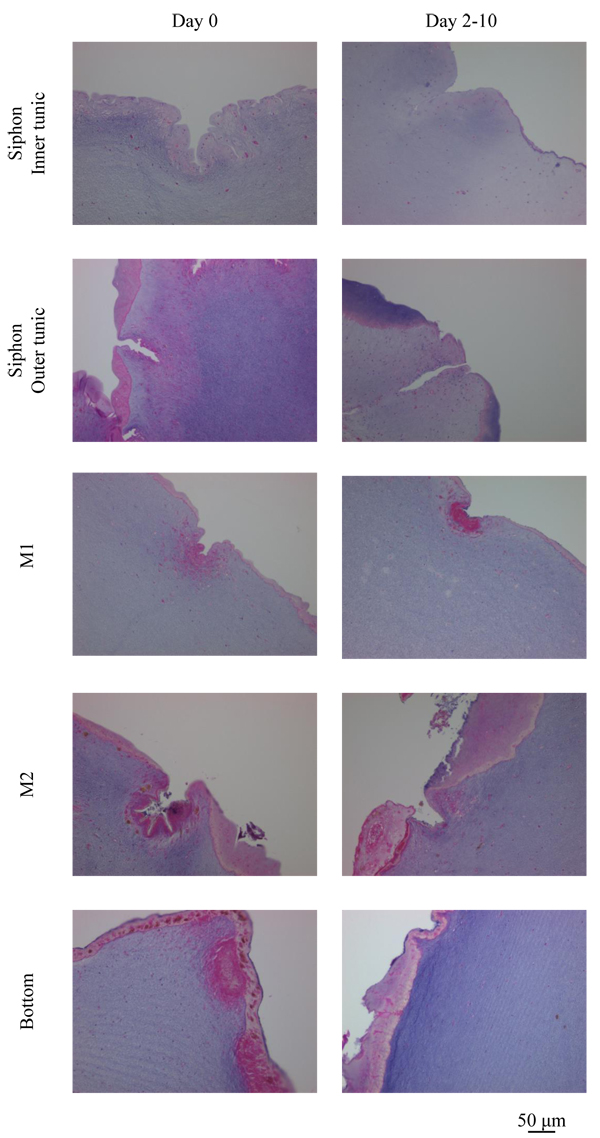 ) shows the outside of the tunic in each region at Day 0 (control) and Day 2-10 (Siphon, Day 5; other regions, Day 10), made by Hematoxylin-Eosin stain. Siphon was divided into two groups: Inner tunic and Outer tunic because the tunic covers the inner and outer surfaces of the tubular muscle. Uneven surfaces were observed in every region at both the divisions of the period (Day 0 and Day 2-10). The characteristics of the layer positive for Hematoxylin-Eosin stain at the outermost surface were diverse in each group but kept at both Day 0 and Day 2-10. The layer at Inner tunic of Siphon was scarcely observed while that at Outer tunic was distributed at the protrusion. At M1, the thin layer at the protrusion and the cells at the dent were observed. The layer at Bottom was relatively thick and distributed entirely. M2 also had the layer, whose characteristics were between those of M1 and Bottom. Fig. (11
) shows the outside of the tunic in each region at Day 0 (control) and Day 2-10 (Siphon, Day 5; other regions, Day 10), made by Hematoxylin-Eosin stain. Siphon was divided into two groups: Inner tunic and Outer tunic because the tunic covers the inner and outer surfaces of the tubular muscle. Uneven surfaces were observed in every region at both the divisions of the period (Day 0 and Day 2-10). The characteristics of the layer positive for Hematoxylin-Eosin stain at the outermost surface were diverse in each group but kept at both Day 0 and Day 2-10. The layer at Inner tunic of Siphon was scarcely observed while that at Outer tunic was distributed at the protrusion. At M1, the thin layer at the protrusion and the cells at the dent were observed. The layer at Bottom was relatively thick and distributed entirely. M2 also had the layer, whose characteristics were between those of M1 and Bottom. Fig. (11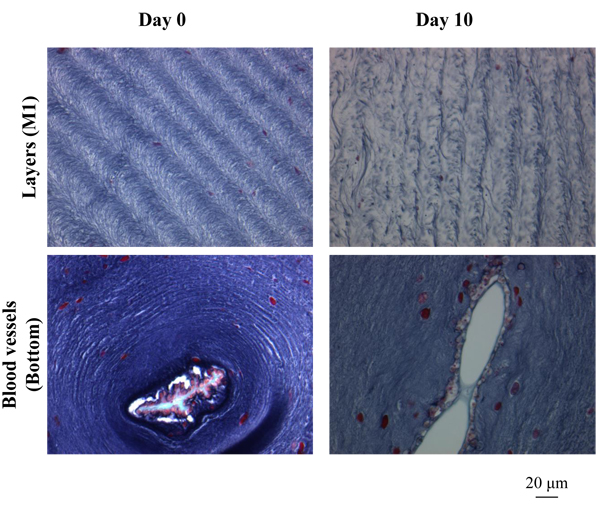 ) shows the characteristic patterns on the inside of the tunic: layers, almost parallel to the outside and winding in some cases, and a blood vessel. The pattern of layers was observed in every region while blood vessels were observed mainly in Bottom, but also in M1 and M2. The distribution of blood vessels agreed with the previous reports [2Berill, N.J. The tunicata, 1950, -4Das, S.M. On the structure and function of the ascidian test. J. Morphol., 1936, 59(3), 589-600.
) shows the characteristic patterns on the inside of the tunic: layers, almost parallel to the outside and winding in some cases, and a blood vessel. The pattern of layers was observed in every region while blood vessels were observed mainly in Bottom, but also in M1 and M2. The distribution of blood vessels agreed with the previous reports [2Berill, N.J. The tunicata, 1950, -4Das, S.M. On the structure and function of the ascidian test. J. Morphol., 1936, 59(3), 589-600.
[http://dx.doi.org/10.1002/jmor.1050590308] ]. These characteristics of the outside and inside tunic were also observed in the specimen receiving the mechanical stimuli at Day 2, whose mass was decreased just after the stimuli were given.
 |
Fig. (11) Histological characteristics of the tunic inside. The characteristic patterns observed in the tunic inside, layers and a blood vessel, are indicated. |
According to Fig. 3 and Table 1, the increment in the mass of the tunic per day, Δmr/day, was different in each region at Day 1-10 and Day 2-10: Siphon > M1; M1 > M2; M2 > Bottom. These characteristics would be reasonable if the layer positive for Hematoxylin-Eosin stain is hard so that it would limit the increase in mass of the inside, or if water hardly penetrates the layer so that influx and efflux of water in the tunic would be limited. Assuming that the blood vessels in the tunic could be collapsed by the change of the mechanical environment, it would be reasonable that Δmr/day at Bottom and Inside were negative.
and Table 1, the increment in the mass of the tunic per day, Δmr/day, was different in each region at Day 1-10 and Day 2-10: Siphon > M1; M1 > M2; M2 > Bottom. These characteristics would be reasonable if the layer positive for Hematoxylin-Eosin stain is hard so that it would limit the increase in mass of the inside, or if water hardly penetrates the layer so that influx and efflux of water in the tunic would be limited. Assuming that the blood vessels in the tunic could be collapsed by the change of the mechanical environment, it would be reasonable that Δmr/day at Bottom and Inside were negative.
4. DISCUSSION
In this study, how the mechanical environment causes the deformation of the tunic was investigated. In order to reduce the activity of the metallo-protease secreted from the hemocytes, the temperatures of the artificial seawater for the specimen in the refrigerator, and at giving the mechanical stimuli to the specimen were 5˚C and less than 10˚C, respectively. The following parameters, which would be associated with the deformation, were evaluated: change in the mass and water content of the tunic, and substances discharged from the tunic, and the histological characteristics of the tunic.
At the tunic specimen without the mechanical stimuli, the increment in the mass of the tunic per day, Δmr/day, was larger as the region became closer to Siphon: Siphon > M1 > M2 > Bottom. In addition, Δmr/day at Inside was significantly lower than that at Outer. However, the difference in Δmr/day between the adjacent regions was not significant when the mechanical stimuli were given to the tunic specimen. Also, the mechanical stimuli reduced Δmr/day at Siphon after the mechanical stimuli were given, but did not reduce those in other regions. The increment in the mass of the tunic was corresponding to that in the mass of water in the tunic whether or not the tunic was mechanically stimulated. As influx and efflux of water were corresponding to increment in the mass of the tunic, the distribution of water in the tunic would be helpful in investigating the movement of water. The method to evaluate the distribution will be developed in the future. The substances discharged from the tunic stimulated mechanically would be also the same as those without the stimuli because the concentration ratio of dissolved organic matter to nitrate was kept regardless of the mechanical stimuli, but the discharge was slowly increased after giving the stimuli to the tunic. This result indicates that the mechanical stimuli could enhance part of functions in the tunic related to the discharged substances slowly. In addition, according to the temperature in this study, the metallo-protease secreted by the hemocyte was less active than that in the previous studies [18Kato, Y. Active movement of the tunic in Halocynthia roretzi. Journal of Biomechanical Science and Engineering, 2010, 5(2), 163-174.
[http://dx.doi.org/10.1299/jbse.5.163] -20Kato, Y. Mechanical senses and the tunic structure in Halocynthia roretzi. In Proceedings of The 2011 International Offshore and Polar Engineering Conference, Maui, Hawaii, USA, June 19-24, 2011; ISOPE: California, USA, 2011; pp.250-253.], but the mechanical stimuli caused a decrease in the mass of the tunic. Substances except for the protease in the tunic would involve the response to the stimuli. The substances, discharged from the tunic regardless of the mechanical environment, will be analyzed in the future. In histological characteristics, the layer positive for Hematoxylin-Eosin stain at the outside was becoming thicker and spreading more widely as the region was closer to Bottom. While the layer pattern in the inside was observed at every region, the blood vessels were mainly observed at the inside of Bottom. Assuming that the layer positive for Hematoxylin-Eosin stain at the outside would hardly deform and indicate higher resistance for the penetration of water than the inside, it would be reasonable that Δmr/day became smaller as the position was closer to Bottom. In addition, the collapse of blood vessels could decrease Δmr/day at both Bottom and Inside. Mechanical characteristics of the layer and the blood vessels should be evaluated by an indentation test in the future. Cells in Inner tunic of Siphon would receive the mechanical stimuli more directly than those in other regions because the layer, positive for Hematoxylin-Eosin stain, scarcely covered the outside. That would make Δmr/day of Siphon at Post lower. The response of the cell to the mechanical environment will be investigated in the future. If Δmr/day at giving the mechanical stimuli is hardly influenced by the difference in the histological characteristics, the amount of substances related to contraction would play an important role in the deformation. Type of the substance related to contraction will be examined in the future study.
CONCLUSION
In this study, how the tunic of Halocynthia roretzi responds to the mechanical environment and deforms itself as result was investigated. In the specimen without the mechanical stimuli, the increment in the mass per day was higher as the region was closer to Siphon, whose outside was scarcely covered with the layer positive for Hematoxylin-Eosin stain. When the tunic received the mechanical stimuli, Siphon was mostly influenced in all the regions. However, the increment in the mass per day at giving the stimuli was not different from that in the adjacent region. Moreover, the increment in the mass was corresponding to influx and efflux of water in the tunic. Substances discharged from the tunic were slowly enhanced by the mechanical stimuli.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Halocynthia roretzi, a solitary ascidian, is an invertebrate species, which legal controls are not imposed on in Japan.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
| [1] | Wertz, J-L.; Bédué, O.; Mercider, J.P. Cellulose science and technology, 2010, |
| [2] | Berill, N.J. The tunicata, 1950, |
| [3] | Goodbody, I. The physiology of ascidians. Advances in Marine Biology., 1974, 12, 1-149. [http://dx.doi.org/10.1016/S0065-2881(08)60457-5] |
| [4] | Das, S.M. On the structure and function of the ascidian test. J. Morphol., 1936, 59(3), 589-600. [http://dx.doi.org/10.1002/jmor.1050590308] |
| [5] | Yokosawa, H.; Sawada, H.; Abe, Y.; Numakunai, T.; Ishii, S. Galactose-specific lectin in the hemolymph of solitary ascidian, Halocynthia roretzi : isolation and characterization. Biochem. Biophys. Res. Commun., 1982, 107(2), 451-457. [http://dx.doi.org/10.1016/0006-291X(82)91512-1] [PMID: 7126223] |
| [6] | Azumi, K.; Yokosawa, H.; Ishii, S. Halocyamines: novel antimicrobial tetrapeptide-like substances isolated from the hemocytes of the solitary ascidian Halocynthia roretzi. Biochemistry, 1990, 29(1), 159-165. [http://dx.doi.org/10.1021/bi00453a021] [PMID: 2322536] |
| [7] | Yokosawa, H.; Harada, K.; Igarashi, K.; Abe, Y.; Takahashi, K.; Ishii, S. Galactose-specific lectin in the hemolymph of solitary ascidian, Halocynthia roretzi. Molecular, binding and functional properties. Biochim. Biophys. Acta, 1986, 870(2), 242-247. [http://dx.doi.org/10.1016/0167-4838(86)90228-1] [PMID: 3006779] |
| [8] | Harada-Azumi, K.; Yokosawa, H.; Ishii, S. N-acetyl-galactosamine-specific lectin, a novel lectin in the hemolymph of the ascidian Halocynthia roretzi: isolation, characterization and comparison with galactose-specific lectin. Comp. Biochem. Physiol. B, 1987, 88B(1), 375-381. [http://dx.doi.org/10.1016/0305-0491(87)90130-1] |
| [9] | Azumi, K.; Yoshimizu, S.; Suzuki, S.; Ezura, Y.; Yokosawa, H. Inhibitory effect of halocyamine, an antimicrobial substance from ascidian hemocytes, on the growth of fish viruses and marine bacteria. Experientia, 1990, 46(10), 1066-1068. [http://dx.doi.org/10.1007/BF01940675] [PMID: 2121517] |
| [10] | Azumi, K.; Yokosawa, H.; Ishii, S. Lipopolysaccharide induces release of a metallo-protease from hemocytes of the ascidian, Halocynthia roretzi. Dev. Comp. Immunol., 1991, 15(1-2), 1-7. [http://dx.doi.org/10.1016/0145-305X(91)90041-V] [PMID: 2050243] |
| [11] | Azumi, K.; Ozeki, S.; Yokosawa, H.; Ishii, S. A novel lipopolysaccharide-binding hemagglutinin isolated from hemocytes of the solitary ascidian, Halocynthia roretzi: it can agglutinate bacteria. Dev. Comp. Immunol., 1991, 15(1-2), 9-16. [http://dx.doi.org/10.1002/jez.1402650312] [PMID: 1904830] |
| [12] | Azumi, K.; Satoh, N.; Yokosawa, H. Functional and structural characterization of hemocytes of the solitary ascidian. Halocynthia roretzi. J. Exp. Zool., 1993, 265, 309-316. [http://dx.doi.org/10.1016/0145-305X(91)90042-W] |
| [13] | Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc., 2002, 124(31), 9074-9082. [http://dx.doi.org/10.1021/ja0257319] [PMID: 12149011] |
| [14] | Zhao, Y.; Li, J. Excellent chemical and material cellulose from tunicates: Diversity in cellulose production yield and chemical and morphological structures from different tunicate species. Cellulose, 2014, 21(5), 3427-3441. [http://dx.doi.org/10.1007/s10570-014-0348-6] |
| [15] | Anno, K.; Otsuka, K.; Seno, N. A chitin sulfate-like polysaccharide from the test of the tunicate Halocynthia roretzi. Biochim. Biophys. Acta, 1974, 362(1), 215-219. [http://dx.doi.org/10.1016/0304-4165(74)90043-9] [PMID: 4421550] |
| [16] | Wagner, G.P. Evolution and multi-functionality of the chitin system. EXS, 1994, 69, 559-577. [http://dx.doi.org/10.1007/978-3-0348-7527-1_33] [PMID: 7994124] |
| [17] | Tuchiya, Y.; Suzuki, Y. Biochemical studies of the ascidian. Cynthia roretzi v. Drasche-VI. Nippon Suisan Gakkaishi, 1962, 28(2), 222-226. [http://dx.doi.org/10.2331/suisan.28.222] |
| [18] | Kato, Y. Active movement of the tunic in Halocynthia roretzi. Journal of Biomechanical Science and Engineering, 2010, 5(2), 163-174. [http://dx.doi.org/10.1299/jbse.5.163] |
| [19] | Kato, Y. The role of protein as a deformation controller in cellulose tissue. In Proceedings of ASME 2012 International Mechanical Engineering Congress and Exposition, Houston, Texas, USA, November 9-15, 2012; ASME: New York, USA, 2012; pp. 607-613 [http://dx.doi.org/10.1115/IMECE2012-89313] |
| [20] | Kato, Y. Mechanical senses and the tunic structure in Halocynthia roretzi. In Proceedings of The 2011 International Offshore and Polar Engineering Conference, Maui, Hawaii, USA, June 19-24, 2011; ISOPE: California, USA, 2011; pp.250-253. |
| [21] | Armstrong, F.A.J.; Boalch, G.T. Volatile organic matter in algal culture media and sea water. Nature, 1960, 185, 761-762. [http://dx.doi.org/10.1038/185761b0] |
| [22] | Armstrong, F.A.J.; Boalch, G.T. The ultra-violet absorption of sea water. J. Mar. Biol. Assoc. U. K., 1961, 41, 591-597. [http://dx.doi.org/10.1017/S0025315400016179] |
| [23] | Ogura, N.; Hanya, T. Nature of ultra-violet absorption of sea water. Nature, 1966, 212, 758. [http://dx.doi.org/10.1038/212758a0] |
| [24] | Ogura, N.; Hanya, T. Ultraviolet absorption of the sea water, in relation to organic and inorganic matters. International Journal of Oceanology and Limnology, 1967, 1(2), 91-102. |
| [25] | Foster, P.; Morris, A.W. The use of ultra-violet absorption measurements for the estimation of organic pollution in inshore sea water. Water Res., 1971, 5, 19-27. [http://dx.doi.org/10.1016/0043-1354(71)90059-5] |
| [26] | Collos, Y.; Mornet, F.; Sciandra, A.; Waser, N.; Larson, A.; Harrison, P.J. An optical method for the rapid measurement of micromolar concentrations of nitrate in marine phytoplankton cultures. J. Appl. Phycol., 1999, 11, 179-184. [http://dx.doi.org/10.1023/A:1008046023487] |












