- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Surface Active Properties and Micellar Features of Copper Soaps Derived from Various Edible Oils
Arun Kumar Sharma1, *, Meenakshi Saxena2, Rashmi Sharma3
Abstract
Introduction:
The molar volume, viscosity, specific viscosity, and fluidity of copper surfactant derived from various edible oils in methanol -benzene solvent have been determined at a constant temperature 303 K.
Methods / Results:
The results were used to calculate (CMC), soap complex-solvent interactions and the effect of chain length of the surfactant molecule on various parameters.
The conclusions with regard to soap-soap and soap- methanol -benzene interaction have been discussed in terms of well-known Moulik’s and Jones- Dole equations. The effect of surfactant concentration on viscosity of the solution in solvent mixture has been discussed.
Conclusion:
The observations suggested that the structure breaking effect by the solute on the solvent molecules is more prominent above CMC as compared to below CMC after the formation of the micelles. The vital information plays an important role in various industrial process as well as biological applications.
Article Information
Identifiers and Pagination:
Year: 2018Volume: 5
First Page: 119
Last Page: 133
Publisher Id: CHEM-5-119
DOI: 10.2174/1874842201805010119
Article History:
Received Date: 11/8/2018Revision Received Date: 20/9/2018
Acceptance Date: 6/11/2018
Electronic publication date: 30/11/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Chemistry, Govt. P.G. College, Jhalawar-326001 (Raj.), India; Tel: +919352669899; E-mail: sharmaarun423@gmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 11-8-2018 |
Original Manuscript | Surface Active Properties and Micellar Features of Copper Soaps Derived from Various Edible Oils | |
1. INTRODUCTION
Surface-active agents are vital components in biological systems and play an important role in many industrial processes because of their ability to lower interfacial tension [1Siddique, J.A.; Sharma, S.; Naqvi, S. Viscometric study of lysozyme solution with sugar and urea at various temperatures. Arab. J. Chem., 2016, 9(2), 1040-1043.
[http://dx.doi.org/10.1016/j.arabjc.2011.11.006] , 2Sharma, A.K.; Saxena, M.; Sharma, R. Fungicidal activities and characterization of novel biodegradable Cu (II) surfactants derived from lauric acid. Open Chem. J., 2018, 5, 89-1051.
[http://dx.doi.org/10.2174/1874842201805010089] ]. Although the use of surfactants in preservation of wood, water proofing and repellency, protection of crops, stabilization of nylon threads, lubrication etc. is relatively a newer applications [3Sharma, A.K.; Sharma, R.; Gangwal, A. Antifungal activities and characterization of some new environmentally safe Cu (II) surfactants substituted 2-amino-6-methyl benzothiazole. Open Phar Sci. J., 2018, 5, 1-11.
[http://dx.doi.org/10.2174/187484490180501] , 4Akhtar, Y. Volumetric and viscometric behaviour of amino acids in aqueous metal electrolytes solutions at 308K. Fluid Phase Equilib., 2007, 258(2), 125-130.
[http://dx.doi.org/10.1016/j.fluid.2007.01.043] ]. Physical properties and biological application in terms of CMC, solvent- solute interactions and fungicidal effect of copper soaps in various organic solvents have been studied by Sharma et al. [5Tank, P.; Sharma, A.K.; Sharma, R. Thermal behaviour and kinetics of copper (II) soaps and complexes derived from mustard and soyabean oil. J. Anal. Pharm. Res., 2017, 4(2), 1-5.
[http://dx.doi.org/10.15406/japlr.2017.04.00102] -10Sharma, S.; Sharma, R.; Sharma, A.K. Photo Catalytic and Kinetic study of ZnO catalyzed degradation of Copper Stearate Surfactant. Current Env. Eng., 2018, 5, 735-745.
[http://dx.doi.org/10.2174/2212717805666180801143324] ]. Sharma et al. have evaluated various parameters pertaining to micellar characteristics i.e. density, apparent molar volume for copper soaps derived from various edible oils [11Tank, P.; Sharma, R.; Sharma, A.K. Micellar features and various interactions of copper soap complexes derived from edible mustard oil in benzene at 303.15 K curr. Phy. Chem., 2018, 8(1), 46-57., 12Bhutra, R.; Sharma, R.; Sharma, A.K. Volumetric studies of copper soap derived from treated and untreated oils in benzene at 298. 15 K Bull. Pure Appl. Sci. Sect. C Chem., 2018, 37(2), 33-4.
[http://dx.doi.org/10.5958/2320-320X.2018.00028.6] ]. A perusal of literature survey reveals that a number of transition metal soaps and their complexes have been studied due to their wide applications, however scanty references are available as to the investigation of copper soap and their complexes in various organic solvents. In our previous communication we report various physical parameters (density, MV, AMV, ultrasound, surface tension) in benzene-methanol mixture (40% and 80%) of copper surfactant complexes of (caprylate, caprate and laurate) with 2-amino, 6-R benzothiazole (R = -Cl, -CH3) [13Sharma, A.K.; Sharma, R.; Gangwal, A.; Das, D. Solute-solvent interaction of ternary system containing copper soap-2-amino-6-chloro benzothiazole complex, benzene and methanol at 298.15 K. Inst. Chem. India, 90, 121-135.], and other physical properties (density, MV, AMV, ultrasound, viscosity)in benzene-methanol mixture (60% and 80%) of copper surfactants derived from neem and karanj oils [14Sharma, A.K. Viscometric Behaviour and Micellar Studies of Cu (II) surfactant derived from Neem (AzadirectaIndica)) oil in methanol-benzene mixture at 298.15 K. Global J. Eng. Sci. Res., 2018, 9-16.]. Mehrotra et al. studied various physical properties of metallic soaps such as Barium soap [15Mehrotra, K.N.; Varma, R.P. Studies on surface tension of the system: Barium soap-water and propanol-1. J. Am. Chem. Soc., 1969, 46(3), 152-154.], samarium soaps [16Mehrotra, K.N.; Chauhan, M.; Shukla, R.K. Surfactants & detergents: Influence of alkanols on the micellar behavior of samarium soaps. J. Am. Oil Chem. Soc., 1990, 67(7), 446-450.
[http://dx.doi.org/10.1007/BF02638959] ], chromium soaps [17Mehrotra, K.N.; Jain, M. Viscometric and spectrophotometric studies of chromium soaps in a benzene—dimethylformamidemixture. Colloids Surf. A Physicochem. Eng. Asp., 1994, 85, 75-80.
[http://dx.doi.org/10.1016/0927-7757(93)02728-W] ], Barium caproate [18Mehrotra, K.N.; Varma, R.P. Studies on the physical properties of the system: Barium caproate*-water and propanol-1. J. Am. Oil Chem. Soc., 1969, 46, 568-592.], manganese Caprylate [19Mehrotra, K.N.; Tonton, K.; Rawat, M.K. Conductivity, viscosity and spectral studies on manganese caprylate in alkanols. Colloids Surf., 1991, 57(1), 125-138.
[http://dx.doi.org/10.1016/0166-6622(91)80185-Q] ], copper soaps [20Mehrotra, K.N.; Mehta, V.P.; Nagar, T.N. Determination of criticalMicelle concentration of copper soaps in non-aqueous solvents. J. Prakt. Chem., 1970, 312, 545-553.
[http://dx.doi.org/10.1002/prac.19703120402] ], and Millard studied Surface Tension parameters of alkaline soap solutions [21Millard, E.B. Surface tension of alkaline soap solutions. Ind. Eng. Chem., 1923, 15(8), 810-811.
[http://dx.doi.org/10.1021/ie50164a016] ]. Mathur et al. studied viscometric behaviour of macromolecular complexes of copper soaps [22Mathur, N.; Bargotya, S.; Mathur, R. Viscometric behaviour and miceller studies of some biodegradable organometallic complexes in binary solvent system. Res. J. Pharm. Biol. Chem. Sci., 2014, 5(2), 989-997.]. The colloidal chemical behaviour of Copper(II) soaps in the non- aqueous mixture of varying composition has been investigated by Mehta et al. using viscometric and allied parameters [23Joram, A.; Sharma, R.; Saxena, A.K. Thermal degradation of complexes derived from Cu (II) groundnut soap (Arachis hypogaea) and Cu (II) sesame soap (Sesamum indicum). Z phys.chem., 2018, 232(4), 459-470.]. Mehrotra et al. carried out versility of colometric and polarographic method for the determination of metal content in copper soaps [24Malik, W.U.; Ahmad, S.I. Studies on the behaviour of chromium (iii) soaps. II. Solubility and viscometric studies with chromium stearate and palmitate in non-aqueous solutions. J. Am. Oil Chem. Soc., 1965, 42, 454-456.
[http://dx.doi.org/10.1007/BF02635591] ]. Malik et al. reported the polarographic behaviour of copper soaps in pyridine, o-toludine and dioxane and the effects of temperature and electrolytes on the solubilization and CMC have been reported [25Mehrotra, K.N.; Mehta, V.P.; Nagar, T.N. Studiesoncolorimetry, solubility and thermodynamicproperties of copper soap solutions. J. Prakt. Chem., 1971, 313, 607-613.
[http://dx.doi.org/10.1002/prac.19713130408] ]. The present work has been initiated with a view to obtain a profile about micellar characterization and aggregation of copper soaps derived from various edible oils in methanol -benzene mixture of 20% and 40% maximum effect of polarity on CMC and other parameters. The physical properties (viz. molar volume, viscosity, specific viscosity, and fluidity) of the copper soaps in “methanol -benzene mixture” have been investigated in order to find out the CMC, nature and size of the micelles formed as well as to test the validity of various equations under different conditions.
2. EXPERIMENTAL
Oils were procured directly from the seeds of Mustard, Groundnut, and Sesame. Soyabean (pure) oil was taken from the market of a reputed brand. The fatty acid composition of oils was confirmed by sending their methyl esters to RSIC, CDRI Lucknow U.P. India (Table 1). Copper soaps were prepared by direct metathesis of the corresponding potassium soap with a slight excess of 50 ml of saturated solution of copper sulphate at 50-550C. After washing with hot water and alcohol, the samples were dried at 100-1050C. Finally under reduced pressure, they were recrystallized twice from hot benzene [26Saxena, M.; Sharma, R.; Sharma, A.K. Micellar Features of Cu (II)Surfactants derived fromEdible Oils., 2017, ]. The synthesized copper soaps shows maximum solubility in benzene and in some organic solvents, the methanol solvent was taken to study the effect of polarity on micellar features and CMC with non -polar benzene. In general, all the four copper soaps green in colour was obtained. Molecular weights of copper soaps was determined from saponification equivalent. The saponification equivalent or saponification value is a measure of the average chain length of the fatty acid which makes up fat. The Saponification Equivalent (S.E) is the amount of potassium hydroxide whilst the Saponification Value (S.V) is the number of milligrams of KOH required to saponify one gram of the oil, the two being related by the expression:
| S.E = 56100/S.V |
S.E may be taken as an average molecular weight of oil. Value of S.E is determined by experiment, the ratio taken for saponification of oil with 2N KOH was 1:10 and from this value, average molecular weight of the copper soap was calculated. According to their molecular formula (RCOO-)2Cu where S.E is taken as average molecular weight of the fatty acid R-COOH which is converting into a soap. The values of S.V., molecular weights and other analytical data are recorded in Table 2.
The systems are abbreviated as follows:
Total 25 ml solution containing different concentrations of solute was prepared in a clean volumetric flask at constant temperature for each study. The densities of pure methanol, benzene was 20% (5ml+20ml) and 40% (10ml+15ml) methanol- benzene solvent mixture. Ubbelohde type viscometer was used for measuring the viscosity of the solutions of varying concentrations of the soap. The densities were determined by means of a Sprengel’s pyknometer. The viscosity of the soap solutions was calculated by the following relationship [27Mehta, V.P.; Hasan, M.; Heda, L.C. Surface tension behavior and micellization of cadmium(II) soap-benzene and methanol at 40°C. J. Macromol. Sci.: Part A - Chem.: Pure Appl. Chem., 1983, 19(2), 171-179.].
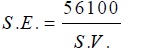 |
(1) |
Where η0,η,ρ0ρ, t0 and t are the viscosity, density and time of flow for the known and unknown solutions, respectively. The accuracy of the results was checked by determining the viscosity of known solutions and the agreement was found to be good and the difference was below 0.3%. All measurements were made at a constant temperature in thermostatic water bath (30+ 0.10C=303K) in a thermostat. The viscosity date was reproducible within ± 0.02 milipoise.
The molar volume of the complex solution ‾V has been calculated by the relationship [28Sharma, A.K.; Saxena, R.; Gangwal, A. Surface tension studies of ternary system: Cu (II) surfactants - 2-amino-6-methyl benzothiazole complex plus methanol plus benzene at 311 K. Curr. phy. Chem., 2018, 8 in press].
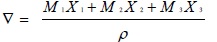 |
(2) |
Where X1 is the mole fraction of the soap of molecular weight M1 whereas X2 is the mole fraction of benzene of molecular weight M2 and X3 is the mole fraction of Methanol of Molecular weight M3 while p stands for density of the solution.The molar volume 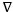 was reproducible within ± m3mol-1
was reproducible within ± m3mol-1
3. RESULTS AND DISCUSSION
3.1. Copper Soaps Derived from Various Edible Oils in 20% Methanol-Benzene Mixture
3.1.1. Molar Volume:
The molar volume 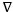 of copper soaps derived from various edible oils in 20% methanol-benzene mixture was calculated from density data and is recorded in Table 3. It has been revealed from Table 3 that the values of molar volume
of copper soaps derived from various edible oils in 20% methanol-benzene mixture was calculated from density data and is recorded in Table 3. It has been revealed from Table 3 that the values of molar volume 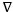 first decreased then there was a slight increase followed by a decrease in a linear manner showing straight line. This may be attributed to the fact that above CMC and in the higher soap concentration region, the degree of aggregation of molecules became dissimilar to that of below CMC. The plots of molar volume, against the soap concentration c are characterized by an intersection of convex curve and a straight line at a definite soap concentration which corresponds to the CMC of the soap. At the CMC, hydrocarbon chain structure of soap molecules allows extensive contact between adjacent chains of fatty acids of varying composition, possibly accompanied by changes in the vibrational and rotational degree of freedom of methylene group.
first decreased then there was a slight increase followed by a decrease in a linear manner showing straight line. This may be attributed to the fact that above CMC and in the higher soap concentration region, the degree of aggregation of molecules became dissimilar to that of below CMC. The plots of molar volume, against the soap concentration c are characterized by an intersection of convex curve and a straight line at a definite soap concentration which corresponds to the CMC of the soap. At the CMC, hydrocarbon chain structure of soap molecules allows extensive contact between adjacent chains of fatty acids of varying composition, possibly accompanied by changes in the vibrational and rotational degree of freedom of methylene group.
The values of CMC so determined are in good agreement with the values obtained from the density measurements. The molar volume of copper soaps derived from various edible oils in 20% methanol-benzene mixture follows the order:
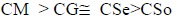 |
The comparison of the results indicates that the values of the molar volume for CM are higher than that of CSo which is due to the presence of longer fatty acid content present in the system.
3.1.2. Viscosity
Flow characterization of solution of soap solutions in terms of viscometric measurements has been employed as a tool to find out CMC of copper soaps. The viscosity, η of copper soaps in non-aqueous solvents of varying composition has been determined at a constant temperature i.e. 30 ±0.10C. The value of viscosity η for all the copper soaps (i.e.CM20, CG20, CSe20, and CSo20) was found to increase with the increase in soap concentration. The increase in viscosity with the increase of soap concentration may be due to the increasing tendency of soap molecules to associate in the form of micelles. The existence of micelles of surfactants in organic solvents and mixed solvents has been reported by number of workers [29Khan, S.; Sharma, R.; Sharma, A.K. Ultrasonic studies of Cu (II) soap derived from seed oil of pongamiapinnata (Karanj), in non-aqueous binary and ternary systems at 298.15K. Malaysian J. Chem., 2017, 19(2), 99-110., 30Sharma, A.K.; Saxena, M.; Sharma, R. Acoustic studies of copper Soap- urea complexes derived from groundnut and sesame oils. J. Phy. Studies., 2017, 21(4), 4601-4606.].The plots of viscosity η against the soap concentration c are characterized by an intersection of two straight lines corresponding to the CMC of the soap Fig. (1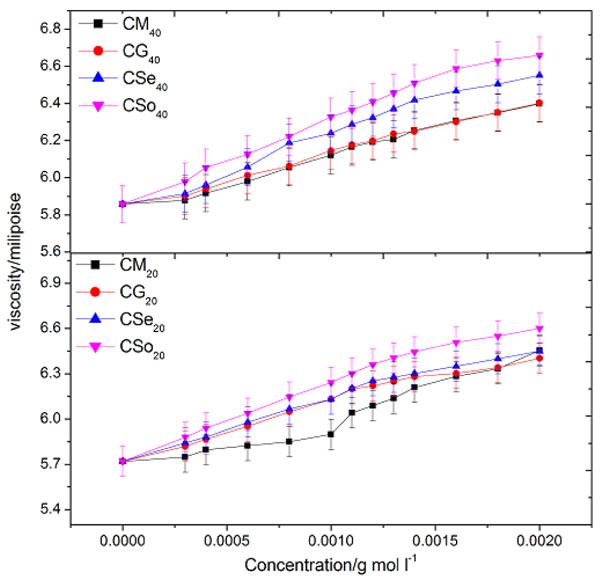 ). The change in the slope of the curve below and above CMC, however, suggests that there is a phenomenal change in the micellar agglomeration below and above CMC. At the CMC, hydrocarbon chain structure of soap molecules allows extensive contact between adjacent chains possibly accompanied by the change in vibration and rotational degree of freedom of methylene group. CMC follows the order:
). The change in the slope of the curve below and above CMC, however, suggests that there is a phenomenal change in the micellar agglomeration below and above CMC. At the CMC, hydrocarbon chain structure of soap molecules allows extensive contact between adjacent chains possibly accompanied by the change in vibration and rotational degree of freedom of methylene group. CMC follows the order:
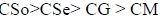 |
The above trend clearly demonstrates that the association of soap molecules occurs at lower concentration in copper soaps of mustard and groundnut soaps in methanol-benzene as compared to sesame and soyabean soaps. It may also be pointed out that the viscosity of the soap solutions decreases with an increase in the chain length of the acid in the soap. This may be due to an increase in the size of the micelles with an increase in the number of carbon atoms in the soap. The viscosity of the soap has been found in the order:
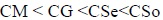 |
The difference in the viscosities of copper soap solutions, in varying composition of methanol-benzene mixture is mainly due to difference in the viscosities of solvent mixture. Thus, with the increase in the number of carbon atoms in the hydrophobic chain, viscosity, η and CMC increased and then decreased. The viscosity results confirm that there is no appreciable aggregation of the soap molecules below the CMC whereas there is a marked change in the aggregation at this soap concentration.
 |
Fig. (1) Plots of viscosity v/s concentration of copper soaps derived from various edible oils in 20 and 40 % methanol- benzene mixture. |
3.1.3. Specific Viscosity
The ratio of change in viscosity to the original viscosity of the solvent is called the specific viscosity ηsp [31Sharma, A.K.; Saxena, M.; Sharma, R. Ultrasonic studies of Cu (II) soaps derived from groundnut and sesame oils. Tenside. Surf. Det., 2017, 55(2), 127-134.
[http://dx.doi.org/10.3139/113.110544] ]
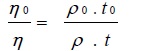 |
(3) |
Where η and η0 are the viscosities of solution and solvent, respectively.
The value of specific viscosity ηsp of copper soaps derived from various edible oils in 20% methanol-benzene mixture was recorded as shown in Table 4. The results show that specific viscosity ηsp of the soap solution increases with the increase in soap concentration. This increase may be due to the increasing tendency of the soap molecules to form aggregates with increasing soap concentration. The values of the CMC so obtained are in good agreement with the values obtained from the plots of viscosity ηagainst the soap concentration c.
3.1.4. Fluidity
The reciprocal of co-efficient of viscosity is called fluidity [32Khan, S.; Sharma, R.; Sharma, A.K. Acoustic studies and other Acoustic Parameters of Cu(II) Soap derived from non- edible Neem oil (Azadirectaindica), in Non-aqueous media at 298.15 Acta. Ac united Ac, 2018, 298, 277-283.
[http://dx.doi.org/10.3813/AAA.919170.] ].
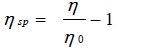 |
(4) |
The fluidityϕ of copper soap solution in 20% methanol-benzene mixture decreases with an increase in soap concentration. The plots of fluidity ϕ against soap concentration (c) show an abrupt change at a point corresponding to the CMC of the soap Fig. (2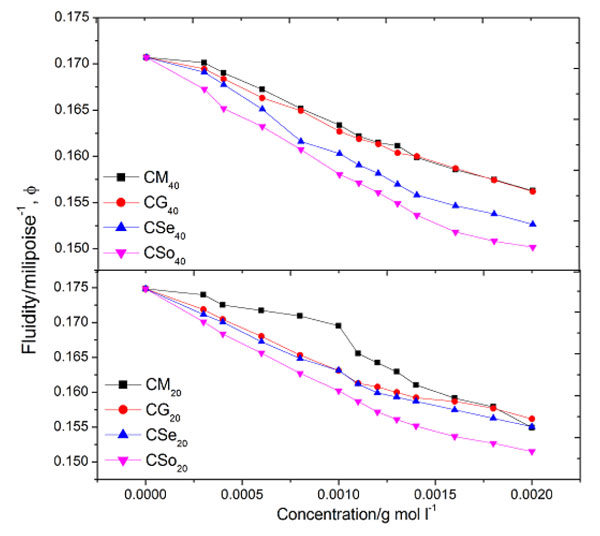 ). A perusal of the CMC data shows that it is dependent on the solvent composition and values are in good agreement with the values obtained from η v/s c, and ηsp v/s c plots.
). A perusal of the CMC data shows that it is dependent on the solvent composition and values are in good agreement with the values obtained from η v/s c, and ηsp v/s c plots.
 |
Fig. (2) Plots of fluidity v/s concentration of copper soaps derived from various edible oils in 20 and 40% methanol- benzene mixture. |
3.1.5. Important Equations
The results of the viscosity of the solutions of copper soaps in 20% methanol-benzene mixture have been interpreted in the light of equations proposed by Einstein, Thomas and Vand.
Einstein Equation [33Einstein, A. A New determination of molecular dimensions. Ann. Phys., 1906, 19, 289-306.
[http://dx.doi.org/10.1002/andp.19063240204] ]:
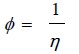 |
(5) |
Thomas equation [34Thomson, D.J. Transport characteristics of suspension: VIII. A note on the viscosity of Newtonian suspensions of uniform spherical particles. J. Colloid Sci., 1965, 20, 267-277.
[http://dx.doi.org/10.1016/0095-8522(65)90016-4] ]:
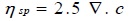 |
(6) |
Vand equation [35Vand, V. Viscosity of solutions and suspensions; theory. J. Phys. Colloid Chem., 1948, 52(2), 277-299.
[http://dx.doi.org/10.1021/j150458a001] [PMID: 18906401] ]
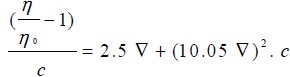 |
(7) |
Where V, c, Q, η,η 0 and ηsp are the molar volume of the soap, concentration of the soap, interaction coefficient, viscosity of the solution and viscosity of the solvent, respectively. The values of molar volume V evaluated from the plots of ηsp v/s c (Einstein equation), ηsp/c η v/s c (Thomas Equation) and 1/c v/s 1/ log η/η0 (Vand equations) was below and above CMC. For our referred system, the plots corresponding to Einstein, Thomas and Vand equation was obtained as an intersection of two straight lines Figs. (3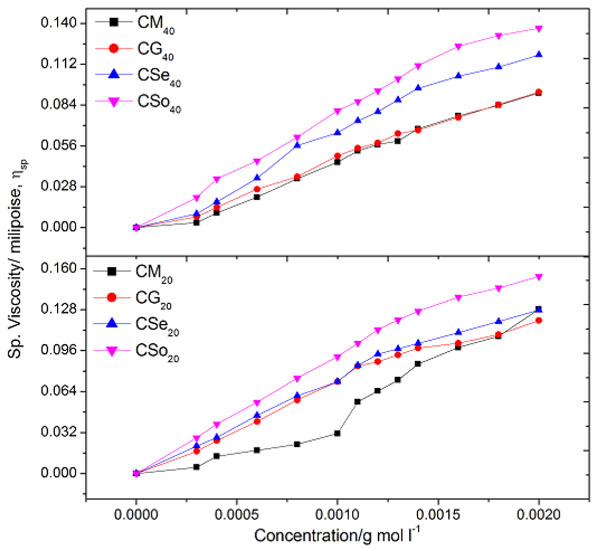 and 5
and 5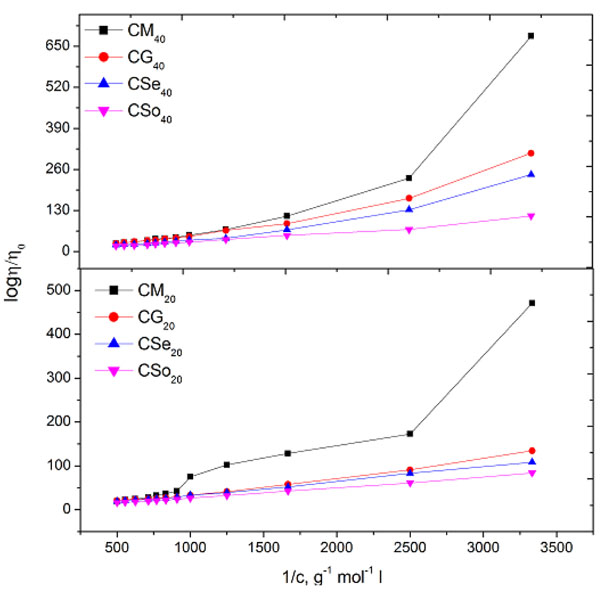 ). Therefore the value of molar volume enumerated from these equations show a slight difference but the trend remains unaltered irrespective of the type of equation applied. Due to irregular trend, Thomas equation is not fit for copper soap for both the solvents i.e.20% methanol and 40% methanol-benzene mixture.
). Therefore the value of molar volume enumerated from these equations show a slight difference but the trend remains unaltered irrespective of the type of equation applied. Due to irregular trend, Thomas equation is not fit for copper soap for both the solvents i.e.20% methanol and 40% methanol-benzene mixture.
 |
Fig. (3) Plots of ηspv/s concentration of copper soaps derived from various edible oils in 20 and 40% methanol- benzene mixture (Einstein Equation). |
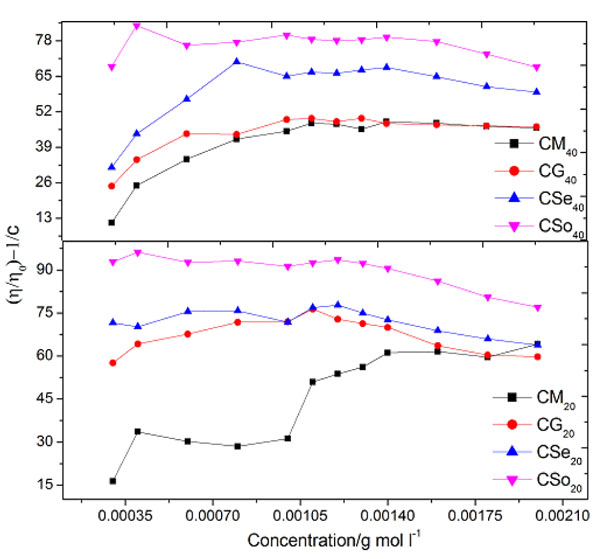 |
Fig. (4) Plots of {(η/η0)-1}/c v/s concentration of copper soaps derived from various edible oils in 20 and 40% methanol- benzene mixture (Thomas Equation). |
 |
Fig. (5) Plots of 1/ {log (η/η0)} v/s 1/c of copper soaps derived from various edible oils in 20 and 40% methanol- benzene mixture (Vand Equation). |
It was observed that the values of molar volume below and above CMC(i.e.V1& V2) enumerated from Einstein equation follow the order Table 5.
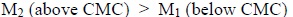 |
Interestingly Moulik’s equation fits equally well to the soap solutions both below and above CMC. The equation is [36Moulik, S.P. Surface potential of aqueous electrolyte solutions. J. Phys. Chem., 1968, 72(2), 74-78.
[http://dx.doi.org/10.1021/j100847a014] ]:
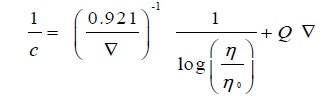 |
(8) |
Where M and K are constants. Evaluated values of M and K from the plots of (η/η 0)2 v/s c2are shown in Table 6.
The plots of (η/η 0)2 v/s c2show a break at a definite soap concentration corresponding to the CMC of the soap Fig. (6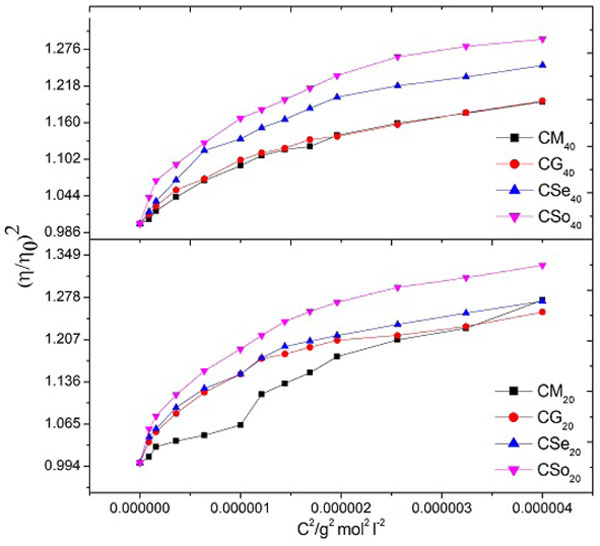 ). The values of Moulik’s constant M and K have been obtained from the intercept and slope of these plots follows the order:
). The values of Moulik’s constant M and K have been obtained from the intercept and slope of these plots follows the order:
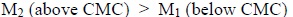 |
Unlike ‘M’, the stipulated values of ‘K’ follow the order:
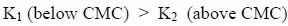 |
 |
Fig. (6) Plots of (η/η 0)2 v/s c2 of copper soaps derived from various edible oils in 20 and 40% methanol- benzene mixture (Moulik’s equation). |
The intrinsic viscosity [η] = limit (c→ 0) [ηsp] of the soap solution according to Gray and Alexander can be represented by the equation [37Gray, V.R.; Alexander, A.E. Viscosity and streaming birefrigence of aluminum soap solutions. J. Phys. Colloid Chem., 1949, 53(1), 9-23.
[http://dx.doi.org/10.1021/j150466a002] [PMID: 18112143] ]:
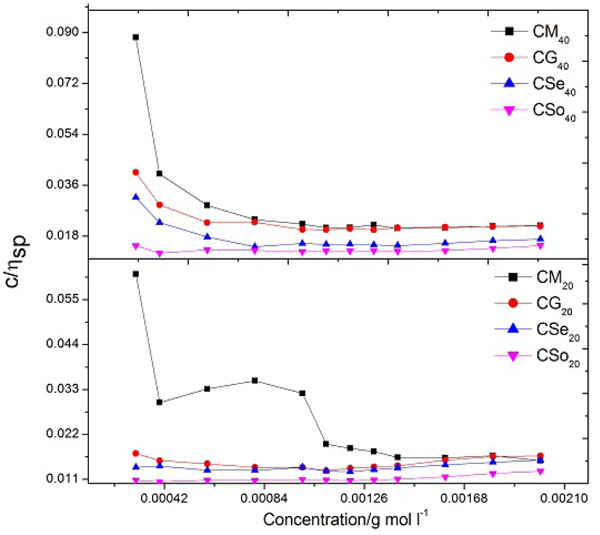 |
Fig. (7) Plots of (c/ηsp) v/s c of copper soaps derived from various edible oils in 20 and 40% methanol- benzene mixture (Gray and Alexander equation). |
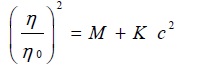 |
(9) |
The values of intrinsic viscosity and coefficient K’ are determined from the plot of reduced viscosity (c/ηsp) against the concentration c of the soap. The plot of (c/ηsp) v/s c is characterized by an intersection of two straight lines Fig. (7 ), thus Gray and Alexander equation fits well both below and above CMC. The sudden change in the trend of the plot at CMC clearly indicated that the change in the behaviour of the soap took place in the periphery of this concentration.
), thus Gray and Alexander equation fits well both below and above CMC. The sudden change in the trend of the plot at CMC clearly indicated that the change in the behaviour of the soap took place in the periphery of this concentration.
The viscosity data have also been interpreted in light of Jones-Dole Equation [38Jones, G.; Dole, M. The viscosity of aqueous solutions of strong electrolytes with special reference to Barium chloride J. Am. Chem. Soc., 1929, 51(1), 2950-2964.
[http://dx.doi.org/10.1021/j150466a002] ]:
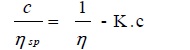 |
(10) |
For convenience, the equation may be expressed as:
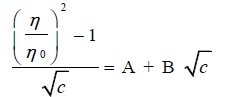 |
(11) |
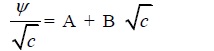 |
(12) |
Where the coefficient A and B refer to the solute–solute and solute–solvent interaction, respectively.
The plots of ψ/√c v/s √c was characterized by an intersection of two straight lines at a point corresponding to the CMC of the soap Fig. (8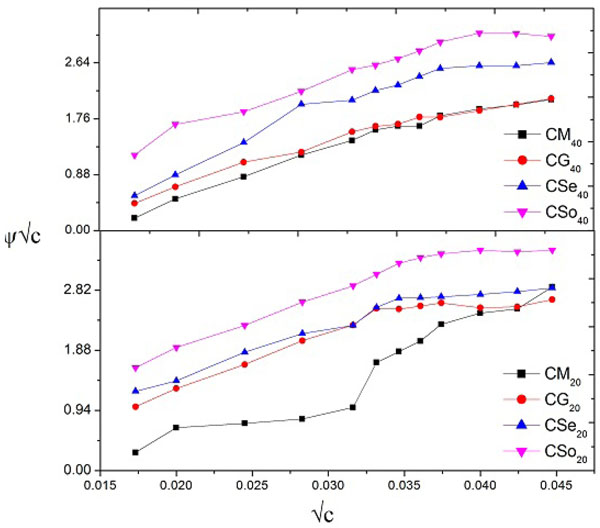 ). The values of CMC are in close agreement with the values obtained fromη v/s c, ηsp v/s c, ϕ v/s c plots.
). The values of CMC are in close agreement with the values obtained fromη v/s c, ηsp v/s c, ϕ v/s c plots.
In view of the two intersecting straight lines forψ/√c v/s √c plots, it is reasonable to evaluate two values of each coefficient below and above CMC designated as A1, B1 and A2, B2, respectively, that are given in Table 6. The positive value of A suggests that there is a strong soap- soap interaction in this system and positive value of B indicates a strong alignment of solvent molecules with solute, which reveals the structure forming behaviour of the solvent.
 |
Fig. (8) Plots of (ψ/√c) v/s √c of copper soaps derived from various edible oils in 20 and 40% methanol- benzene mixture (Jones – Dole equation). |
From the data collected it is obvious that below the CMC, the values of coefficient B1 (soap-solvent interaction) are larger than the values of coefficient A1 (soap-soap interaction) which confirms that the molecules of the soap do not aggregate appreciably below the CMC and there is sudden change in the aggregation above the CMC. From this trend, it is clear that soap-solvent interaction is larger than the soap-soap interaction in dilute solution. The trend of these coefficients may be as follows:
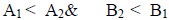 |
From this trend it may be concluded that soap-solvent interaction is more pronounced below CMC. This may be ascribed to the favorable interaction between soap and solvent molecules at the premicellar concentration.
3.2. Copper Soaps Derived from Various Edible Oils in 40% Methanol-Benzene Mixture
3.2.1. Molar Volume
The value of molar volume forcopper soaps derived from various edible oils in 40% methanol-benzene mixture were calculated and recorded. The results show that the effect of soap concentration on the molar volume of copper soap solutions in 40% methanol-benzene mixture is almost similar to that of molar volume of copper soap solutions in 20% methanol-benzene mixture.
The plots of molar volume, V against the soap concentration is characterized by an intersection of convex curve and a straight line. Below CMC, a straight line followed by a convex curve in increasing trend is observed, and after CMC, there is a sharp decrease in molar volume.
The values of CMC determined are in good agreement with the values obtained from density measurements. The values of molar volume V for 40% methanol-benzene mixture was observed lower than that of 20% methanol-benzene mixture which is due to the fact that the nature of solvent plays a significant role in ordering and compacting the scattered micellar units. It is further suggested that methanol occupies quite a different position in the palisade layers of soap anion exhibiting a different degree of aggregation in the mixed solvent of varying composition.
3.2.2. Viscosity, Specific Viscosity, and Fluidity
The effect of the soap concentration on the viscosity, specific viscosity and fluidity of copper soap in 40% methanol-benzene mixture of varying composition similar to that of 20% methanol-benzene mixture. It observed that the viscosity of copper soap in 40% methanol-benzene mixture slightly higher than 20% methanol-benzene mixture which is due to the difference in the viscosities of solvent mixtures. The values of the CMC for the above referred system are recorded in Table 7. It can be easily seen from the table that the CMC of these soap follows the order:
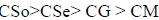 |
This is an agreement with the fact that there is a decrease in the CMC values with the increase in the number of carbon atom in the hydrophobic chain. Viscosity of copper soap derived from various edible oils in 40% methanol-benzene follows the order:
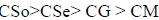 |
The above trend clearly demonstrates that the viscosity of the soap solutions increases with an increase in the average molecular weight of the soap.
3.2.3. Important Equations
It has been found that Einstein and Vand’s equations are equally applicable to copper soap solution in 40% methanol-benzene mixture. But Thomas equation gives no plausible inference due to irregular data. The change in the values of molar volume below and above CMC may due to the fact that entrapping of solvent molecules below CMC is dissimilar to the entrapping of solvent molecules above CMC. From comparison of the result of copper soap in both the solvents, it maybe suggested that the values of molar volume was higher in 20% methanol-benzene mixture than that of 40% methanol-benzene mixture.
Furthermore, the viscometric data below and above CMC have also been interpreted in terms of Moulik’s equation and pertaining parameters M and K. The order of M and K below and above CMC follows the order:
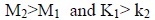 |
Jones-Dole equation is also applicable to copper soap in 40% methanol-benzene mixture. The values of A1, A2 and B1, B2 was calculated and the trend observed similar to that of copper soap in 20% methanol-benzene mixture.
A review of the results shows that below the CMC, the value of coefficient B1 is larger than the value of coefficient A1. This trend indicates that below the CMC, the solute-solvent interaction is more pronounced than solute-solute interaction. The trend of these coefficient is shown below-
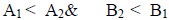 |
The order A2 > A1 is inconsonance with the expectations that solute-solute interaction becomes stronger after CMC as the micellar aggregation takes place. For all the copper soap, the positive values indicate a strong alignment of solvent molecules which reveals the structure forming the behavior of solvent.
Literature survey [39Tank, P.; Sharma, R.; Sharma, A.K. Studies of Ultrasonic and acoustic parameters of complexes derived from Copper (II) surfactant of mustard oil with N and S atoms containing ligands in non- aqueous media (benzene) at 303.15 K. J. Acous. Soc. Ind., 2017, 44(2), 87-99., 40Bhutra, R.; Sharma, R.; Sharma, A.K. Viscometric and CMC studies of Cu(II) surfactants derived from untreated and treated groundnut and mustard oils in non-aqueous solvent at 298.15 K J. Inst. Chemists (India), 2017, 90, 29-47.] reveals that the positive value of A suggests a strong solute-solute interaction. In our referred system, the values of A 1 and A2 are positive and they follow the orderA1<A2. Thus, it is suggested that solute-solute interaction becomes stronger after CMC as the micellar aggregation takes place as compared to before CMC.
Further A1 and A2 (solute-solute) interaction in 20% methanol-benzene mixture was found higher than in 40% methanol-benzene mixture. This may be accounted in terms of change in the mobility of the solute with a change in the dielectric constant of the medium. The positive value of B indicated a strong alignment of solvent molecules with solute, which reveals the structure forming behavior of the solvent.
The decrease in B values B1(40%) < B1 (20%) showed that solute-solvent interactions are influenced by the increase in the non-polar solvent content in the system below and above CMC.
CONCLUSION
The viscosity parameters are important to understand the colloidal behavior, CMC characteristics and nature of the complexes. These studies indirectly help in identifying the structural insight, physical and biochemical properties of above metallic soaps. The present research work makes an attempt to prepare surface active compounds from metal and natural oils. It has been found that the beneficial effects of the synthesized biologically active molecules, agrochemicals and pharmaceuticals are still open for further research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors pay their sincere gratitude to UGC for Financial support, Principal, S. D. Govt. College, Beawar, S.P.C. Govt. College Ajmer Rajasthan (India) for providing necessary research facilities to accomplish this study.
REFERENCES
| [1] | Siddique, J.A.; Sharma, S.; Naqvi, S. Viscometric study of lysozyme solution with sugar and urea at various temperatures. Arab. J. Chem., 2016, 9(2), 1040-1043. [http://dx.doi.org/10.1016/j.arabjc.2011.11.006] |
| [2] | Sharma, A.K.; Saxena, M.; Sharma, R. Fungicidal activities and characterization of novel biodegradable Cu (II) surfactants derived from lauric acid. Open Chem. J., 2018, 5, 89-1051. [http://dx.doi.org/10.2174/1874842201805010089] |
| [3] | Sharma, A.K.; Sharma, R.; Gangwal, A. Antifungal activities and characterization of some new environmentally safe Cu (II) surfactants substituted 2-amino-6-methyl benzothiazole. Open Phar Sci. J., 2018, 5, 1-11. [http://dx.doi.org/10.2174/187484490180501] |
| [4] | Akhtar, Y. Volumetric and viscometric behaviour of amino acids in aqueous metal electrolytes solutions at 308K. Fluid Phase Equilib., 2007, 258(2), 125-130. [http://dx.doi.org/10.1016/j.fluid.2007.01.043] |
| [5] | Tank, P.; Sharma, A.K.; Sharma, R. Thermal behaviour and kinetics of copper (II) soaps and complexes derived from mustard and soyabean oil. J. Anal. Pharm. Res., 2017, 4(2), 1-5. [http://dx.doi.org/10.15406/japlr.2017.04.00102] |
| [6] | Bhutra, R.; Sharma, R.; Sharma, A.K. Synthesis, characterization and fungicidal activities of Cu (II) surfactants derived from groundnut and mustard oils treated at high temperatures. J. Inst. Chemists (India), 2018, 90(3), 66-80. |
| [7] | Bhutra, R.; Sharma, R.; Sharma, A.K. Fungicidal activities of Cu (II) soaps derived from various oils treated at high temperature for biomedical use. SAJ Biotechnol., 2018, 5(103), 1-6. |
| [8] | Sharma, A.K.; Sharma, R.; Saxena, M. Saxena (2018) Biomedical and antifungal application of Cu(II) soaps and its urea complexes derived from various oils.. Open Access J. Trans Med. Res., 2018, 2(2), 40-43. [http://dx.doi.org/10.15406/oajtmr.2018.02.00033] |
| [9] | Mathur, N.; Jain, N.; Sharma, A.K.; Tank, P.; Sherwani, M.R.K. Biocidal activities of substituted benzothiazole of copper surfactants overCandida albicans & Trichoderma harzianumon Muller Hinton Agar. Open Phar Sci. J., 2018, 5, 24-35. |
| [10] | Sharma, S.; Sharma, R.; Sharma, A.K. Photo Catalytic and Kinetic study of ZnO catalyzed degradation of Copper Stearate Surfactant. Current Env. Eng., 2018, 5, 735-745. [http://dx.doi.org/10.2174/2212717805666180801143324] |
| [11] | Tank, P.; Sharma, R.; Sharma, A.K. Micellar features and various interactions of copper soap complexes derived from edible mustard oil in benzene at 303.15 K curr. Phy. Chem., 2018, 8(1), 46-57. |
| [12] | Bhutra, R.; Sharma, R.; Sharma, A.K. Volumetric studies of copper soap derived from treated and untreated oils in benzene at 298. 15 K Bull. Pure Appl. Sci. Sect. C Chem., 2018, 37(2), 33-4. [http://dx.doi.org/10.5958/2320-320X.2018.00028.6] |
| [13] | Sharma, A.K.; Sharma, R.; Gangwal, A.; Das, D. Solute-solvent interaction of ternary system containing copper soap-2-amino-6-chloro benzothiazole complex, benzene and methanol at 298.15 K. Inst. Chem. India, 90, 121-135. |
| [14] | Sharma, A.K. Viscometric Behaviour and Micellar Studies of Cu (II) surfactant derived from Neem (AzadirectaIndica)) oil in methanol-benzene mixture at 298.15 K. Global J. Eng. Sci. Res., 2018, 9-16. |
| [15] | Mehrotra, K.N.; Varma, R.P. Studies on surface tension of the system: Barium soap-water and propanol-1. J. Am. Chem. Soc., 1969, 46(3), 152-154. |
| [16] | Mehrotra, K.N.; Chauhan, M.; Shukla, R.K. Surfactants & detergents: Influence of alkanols on the micellar behavior of samarium soaps. J. Am. Oil Chem. Soc., 1990, 67(7), 446-450. [http://dx.doi.org/10.1007/BF02638959] |
| [17] | Mehrotra, K.N.; Jain, M. Viscometric and spectrophotometric studies of chromium soaps in a benzene—dimethylformamidemixture. Colloids Surf. A Physicochem. Eng. Asp., 1994, 85, 75-80. [http://dx.doi.org/10.1016/0927-7757(93)02728-W] |
| [18] | Mehrotra, K.N.; Varma, R.P. Studies on the physical properties of the system: Barium caproate*-water and propanol-1. J. Am. Oil Chem. Soc., 1969, 46, 568-592. |
| [19] | Mehrotra, K.N.; Tonton, K.; Rawat, M.K. Conductivity, viscosity and spectral studies on manganese caprylate in alkanols. Colloids Surf., 1991, 57(1), 125-138. [http://dx.doi.org/10.1016/0166-6622(91)80185-Q] |
| [20] | Mehrotra, K.N.; Mehta, V.P.; Nagar, T.N. Determination of criticalMicelle concentration of copper soaps in non-aqueous solvents. J. Prakt. Chem., 1970, 312, 545-553. [http://dx.doi.org/10.1002/prac.19703120402] |
| [21] | Millard, E.B. Surface tension of alkaline soap solutions. Ind. Eng. Chem., 1923, 15(8), 810-811. [http://dx.doi.org/10.1021/ie50164a016] |
| [22] | Mathur, N.; Bargotya, S.; Mathur, R. Viscometric behaviour and miceller studies of some biodegradable organometallic complexes in binary solvent system. Res. J. Pharm. Biol. Chem. Sci., 2014, 5(2), 989-997. |
| [23] | Joram, A.; Sharma, R.; Saxena, A.K. Thermal degradation of complexes derived from Cu (II) groundnut soap (Arachis hypogaea) and Cu (II) sesame soap (Sesamum indicum). Z phys.chem., 2018, 232(4), 459-470. |
| [24] | Malik, W.U.; Ahmad, S.I. Studies on the behaviour of chromium (iii) soaps. II. Solubility and viscometric studies with chromium stearate and palmitate in non-aqueous solutions. J. Am. Oil Chem. Soc., 1965, 42, 454-456. [http://dx.doi.org/10.1007/BF02635591] |
| [25] | Mehrotra, K.N.; Mehta, V.P.; Nagar, T.N. Studiesoncolorimetry, solubility and thermodynamicproperties of copper soap solutions. J. Prakt. Chem., 1971, 313, 607-613. [http://dx.doi.org/10.1002/prac.19713130408] |
| [26] | Saxena, M.; Sharma, R.; Sharma, A.K. Micellar Features of Cu (II)Surfactants derived fromEdible Oils., 2017, |
| [27] | Mehta, V.P.; Hasan, M.; Heda, L.C. Surface tension behavior and micellization of cadmium(II) soap-benzene and methanol at 40°C. J. Macromol. Sci.: Part A - Chem.: Pure Appl. Chem., 1983, 19(2), 171-179. |
| [28] | Sharma, A.K.; Saxena, R.; Gangwal, A. Surface tension studies of ternary system: Cu (II) surfactants - 2-amino-6-methyl benzothiazole complex plus methanol plus benzene at 311 K. Curr. phy. Chem., 2018, 8 in press |
| [29] | Khan, S.; Sharma, R.; Sharma, A.K. Ultrasonic studies of Cu (II) soap derived from seed oil of pongamiapinnata (Karanj), in non-aqueous binary and ternary systems at 298.15K. Malaysian J. Chem., 2017, 19(2), 99-110. |
| [30] | Sharma, A.K.; Saxena, M.; Sharma, R. Acoustic studies of copper Soap- urea complexes derived from groundnut and sesame oils. J. Phy. Studies., 2017, 21(4), 4601-4606. |
| [31] | Sharma, A.K.; Saxena, M.; Sharma, R. Ultrasonic studies of Cu (II) soaps derived from groundnut and sesame oils. Tenside. Surf. Det., 2017, 55(2), 127-134. [http://dx.doi.org/10.3139/113.110544] |
| [32] | Khan, S.; Sharma, R.; Sharma, A.K. Acoustic studies and other Acoustic Parameters of Cu(II) Soap derived from non- edible Neem oil (Azadirectaindica), in Non-aqueous media at 298.15 Acta. Ac united Ac, 2018, 298, 277-283. [http://dx.doi.org/10.3813/AAA.919170.] |
| [33] | Einstein, A. A New determination of molecular dimensions. Ann. Phys., 1906, 19, 289-306. [http://dx.doi.org/10.1002/andp.19063240204] |
| [34] | Thomson, D.J. Transport characteristics of suspension: VIII. A note on the viscosity of Newtonian suspensions of uniform spherical particles. J. Colloid Sci., 1965, 20, 267-277. [http://dx.doi.org/10.1016/0095-8522(65)90016-4] |
| [35] | Vand, V. Viscosity of solutions and suspensions; theory. J. Phys. Colloid Chem., 1948, 52(2), 277-299. [http://dx.doi.org/10.1021/j150458a001] [PMID: 18906401] |
| [36] | Moulik, S.P. Surface potential of aqueous electrolyte solutions. J. Phys. Chem., 1968, 72(2), 74-78. [http://dx.doi.org/10.1021/j100847a014] |
| [37] | Gray, V.R.; Alexander, A.E. Viscosity and streaming birefrigence of aluminum soap solutions. J. Phys. Colloid Chem., 1949, 53(1), 9-23. [http://dx.doi.org/10.1021/j150466a002] [PMID: 18112143] |
| [38] | Jones, G.; Dole, M. The viscosity of aqueous solutions of strong electrolytes with special reference to Barium chloride J. Am. Chem. Soc., 1929, 51(1), 2950-2964. [http://dx.doi.org/10.1021/j150466a002] |
| [39] | Tank, P.; Sharma, R.; Sharma, A.K. Studies of Ultrasonic and acoustic parameters of complexes derived from Copper (II) surfactant of mustard oil with N and S atoms containing ligands in non- aqueous media (benzene) at 303.15 K. J. Acous. Soc. Ind., 2017, 44(2), 87-99. |
| [40] | Bhutra, R.; Sharma, R.; Sharma, A.K. Viscometric and CMC studies of Cu(II) surfactants derived from untreated and treated groundnut and mustard oils in non-aqueous solvent at 298.15 K J. Inst. Chemists (India), 2017, 90, 29-47. |