- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Removal of Zinc (II) and Ni (II) by using Bio-Polymer “Chitin”
Baby AbrarUnnisa Begum1, N. Devanna2, *, P. Ramesh Chandra3, Razia Sultana4
Abstract
Introduction:
The increasing level of toxic metals in the environment is causing serious repercussions on the health of human beings. To mitigate the adverse effects of heavy metal concentration in the receiving waters, it is necessary to develop an affordable cost effective technology, which is effective to remove the heavy metals. The purpose of the present work is to investigate the removal of Nickel and Zinc ions from the water using known adsorbents. Chitin is a biopolymer used as as adsorbent to remove metal ions from the solution. Chitin is inexpensive adsorbent and is available abundantly.
Methods and Materials:
In this study, the adsorption techniques, such as variations at different pH levels, contact time and dosage of known concentration of different metal standard solutions and quantity of adsorbent were carried out. The experimental data is attached overleaf with different isotherms and kinetic models.
Results:
The results revealed that the optimum pH found in the removal of Zn and Nickel ions is 6-6.5. The equilibrium attained after 7 minutes. The low pore size was observed to be effective for adsorption. The results of the experimental data revealed that the adsorption process follows the first order kinetic model. By using Langmuir isotherm, the adsorption capacity of Chitin was evaluated.
Conclusion:
The results indicate that the Chitin is one of the best adsorbents for the removal of cations from the aqueous solution.
Article Information
Identifiers and Pagination:
Year: 2018Volume: 5
First Page: 172
Last Page: 181
Publisher Id: CHEM-5-172
DOI: 10.2174/1874842201805010172
Article History:
Received Date: 23/7/2018Revision Received Date: 7/9/2018
Acceptance Date: 7/11/2018
Electronic publication date: 31/12/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Chemistry, JNTUA, Anantapuram, A.P., India; Tel: 9440580507; E-mail: devanna.chemistry@jntu.ac.in
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 23-7-2018 |
Original Manuscript | Removal of Zinc (II) and Ni (II) by using Bio-Polymer “Chitin” | |
1. INTRODUCTION
The rapid growth of industrialization and urbanization started the use of large quantities of heavy metals resulting in the contamination of environment [1Bradl, H., Ed.; Heavy Metals in the Environment: Origin, interaction and remediation., 2002, , 6.]. Heavy metal pollution is an important problem, particularly in water owing to its hazardous/toxic effects and bio- accumulation nature in the food chain of human body [2Kapoor, A.; Viraraghavan, J Fungal bio sorption an alternative option for heavy metal bearing waste water. A Review in Bio Resource Technology, 1995, 53(3), 195-206.]. The main source of heavy metal pollution (Zn and Ni) in the water drives from electroplating industries [3Ramya, R.; Sankar, P.; Anbalagan, S.; Sudha, P.N. Adsorption of Ni(II) from metal solution using cross linked chitosan-g-acrylonitrile copolymer. International Journal of Environmental Sciences, 2001, 1(6), 1323-1335., 4Ravikumar, M.N.V. A review of chitin and chitosan application. React. Funct. Polym., 2000, 46, 1-27.
[http://dx.doi.org/10.1016/S1381-5148(00)00038-9] ]. industrial processes such as galvanization, mining, and melting. Heavy metals are non-degradable and potentially hazardous. Hence, the removal of heavy metals like Nickel and Zinc is highlighted in this study. Nickel and Zinc were chosen for this study, because of its widespread use in industries. it has remarkable water pollution effect and constant vulnerability to the Nickel this creates lung cancer and malfunction of kidneys. Exposure of zinc may cause skin irritation, vomiting, nausea and stomach cramps. Zinc is toxic with the permissible limit of 5 mg/l concentration. Nickel carbonyl is highly toxic compound in nature [5M, Sudha. P.; Gakwisiri, C.; Raut, N.; Al-Saadi, A.; Al-Aisri, S.; Al-Ajmi, A. A critical review of removal of zinc from wastewater Proceedings of the World Congress on Engineering, 2012, 1, 627-630.].
Many conventional methods are being applied for the removal of soluble metals in water by oxidation, reduction, ion exchange, membrane filter and adsorption [6Wasewar, K.L.; Mohammad, A.; Prasad, B.; Mishra, I.M. Adsorption of Zn using factory tea waste: Kinetics, equilibrium a nd thermodynamics. CLEAN: Soil. Water. Air, 2008, 36(3), 320-329.
[http://dx.doi.org/10.1002/clen.200700139] ]. Adsorption is regarded as dependable and effective technique to get rid of Zinc and Nickel from water owing to high efficiency at lower concentration, easy handling and cost-effective.
Various studies have been carried out to create suitable sorbents/adsorbents/bio-sorbents for removal of heavy metals from aqueous solution [7Crini, G.; Peindy, H.N.; Gimbert, F.; Robert, C. Removal of C.I. basic green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: Kinetic and equilibrium studies. Separ. Purif. Tech., 2007, 53, 97-110.
[http://dx.doi.org/10.1016/j.seppur.2006.06.018] ]. One such adsorbent is Chitin, which is effective in a low concentration of heavy metals. Chitin is a biopolymer (Hexoamines) with molecular weight of 203.2 g/mol. Chitin is a fibrous substance consisting of polysaccharides, which is major constituent in the exoskeleton of arthropods such as crustaceans (crabs, lobsters and shrimps) and cell walls of fungi [8Gupta, V.K.; Shrivastava, A.K.; Jain, N. Biosorption of Chromium(VI) from aqueous solutions by green algae Spirogyra species. Water Res., 2001, 35(17), 4079-4085.
[http://dx.doi.org/10.1016/S0043-1354(01)00138-5] [PMID: 11791837] ].
2. MATERIALS AND METHODS
In the present study, Chitin was applied for removal of Nickel and Zinc from the groundwater [9Roberts, G.A.F. Chitin Chemistry., 1992,
[http://dx.doi.org/10.1007/978-1-349-11545-7] ]. This study was carried out at various variables like pH levels, initial concentration, contact time and dosage of Chitin for removal of Ni (II) and Zn (II) from aqueous solution [10Koumanova, B.; Peeva, P.; Allen, S.J.; Gallgher, K.A.; Healy, M.G. Biosorption from aqueous solutions by egg shell membranes and rhizopus oryzae: Equilibrium and Kinetic studies. J. Chem. Technol. Biotechnol., 2002, 77, 539-545.
[http://dx.doi.org/10.1002/jctb.601] , 11Sampranpiboon, P.; Charnkeitkong, P. Equilibrium Isotherm, hermodynamic and kinetic studies of lead adsorption onto pineapple and paper wastes. Int. J. Energy Environ., 2010, 4, 88-97.]. Based on the results, adsorption isotherm and kinetics of adsorption were investigated.
YU et al. reports that the metals can be removed by polymerized saw dust with equilibrium of 3 hrs. In order to reduce the equilibrium time, Chitin was used as physical adsorbent for the removal of heavy metal ions in the present case study [12Bolto, B.A. Soluble polymers in water purification. Prog. Polym Sci., 1995, 20, 987-1041., 13a) Chiou, M.S.; Li, H.Y. Equilibrium and kinetic modeling of adsorption of reactive dye on cross-linked chitosan beads. J. Hazard. Mater., 2002, 93(2), 233-248.
[http://dx.doi.org/10.1016/S0304-3894(02)00030-4] [PMID: 12117469] b) Raut, N.; Charif, G. A critical review of removal of zinc from wastewater. Proceedings of the world congress on engineering, 2012, 4-6.] (Fig. 1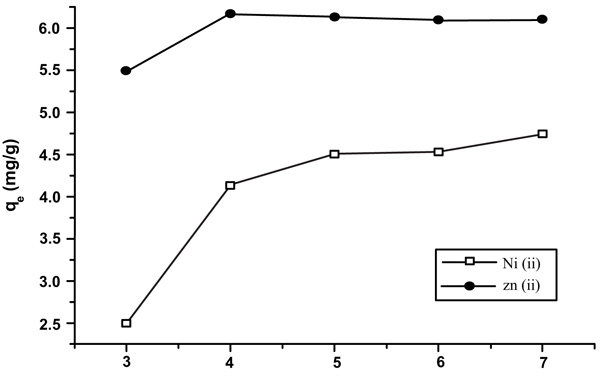 ).
).
 |
Fig. (1) Equilibrium time of 7 min shows qe (mg/g) versus time. |
3. RESULT AND DISCUSSION
3.1. pH Effects
To establish a optimum pH range for the removal of Zinc and Nickel ions, the surface phenomena studies were taken up over pH range of 2-9.5. It was noticed that with the increased pH of the solution and remained nearly constant at higher pH values. With the increase of pH, the percentage of adsorption increased. With Zinc, the removal is 66.9% to 71.9% and a pH increase from 4.0 to 8.0 (Figs. 2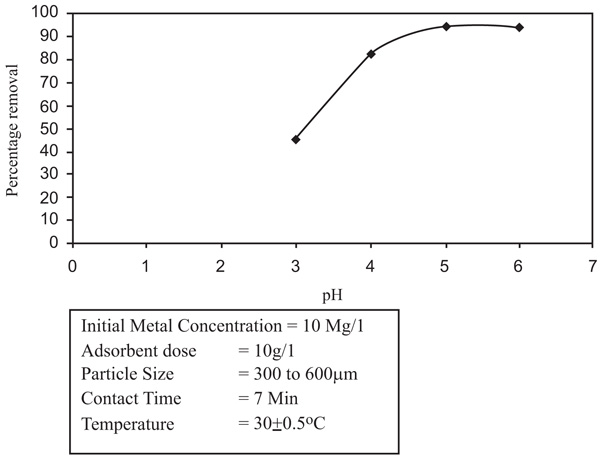 and 3
and 3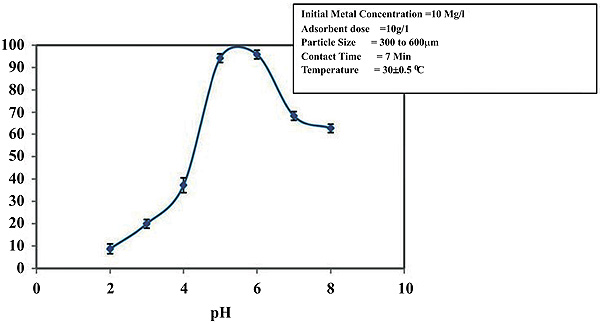 ). In the situation of Nickel, the removal increased from 62.7% to 85.5% with an increase in the pH from 4.5 to 9.5. So the removal of nickel enhanced the scale of pH 4.5 to 6.5 and stabilized at pH 6.5. The removal of nickel was marginal with low pH values because the number of hydrogen ions increased which has the ability to compete with metal ions thus decreasing the efficiency of adsorption. The optimum pH for zinc and Nickel removal is 6.0 and 6.5 respectively.
). In the situation of Nickel, the removal increased from 62.7% to 85.5% with an increase in the pH from 4.5 to 9.5. So the removal of nickel enhanced the scale of pH 4.5 to 6.5 and stabilized at pH 6.5. The removal of nickel was marginal with low pH values because the number of hydrogen ions increased which has the ability to compete with metal ions thus decreasing the efficiency of adsorption. The optimum pH for zinc and Nickel removal is 6.0 and 6.5 respectively.
 |
Fig. (2) Effect of pH on removal of Zinc. |
 |
Fig. (3) Effect of pH on removal of Nickel. |
3.2. Effects of Agitation Time and Initial Concentration
Removal of Zinc and Nickel from aqueous solution by adsorption phenomena on chitin increases with time till equilibrium is attained. It was witnessed that an initial remarkable uptake was indicated by the high initial slope of curves. It occurred owing to the fact that in the beginning, the adsorption was empty, hence the percent of removal is high. After the rapid initial uptake, there is a transitional phase in which the rate of uptake is decreased up To a certain level of concentration and reached at a constant value. The time of equilibrium for both the metals is the same i.e. 7 minutes. As the initial concentration of the nickel increased in the range of 5 to 15 mg/l [14Elangovan, R.; Philip, L.; Chandraraj, K. Biosorption of chromium species by aquatic weeds: Kinetics and mechanism studies. J. Hazard. Mater., 2008, 152(1), 100-112.
[http://dx.doi.org/10.1016/j.jhazmat.2007.06.067] [PMID: 17689012] ], there is an increase in the adsorbed from 0.385 to 1.30 mg/gm. In the case of Zinc [15Abdelwahab, O. Kinetic and isotherm studies of copper (II) removal from wastewater using various adsorbents. Egypt. J. Aquat. Res., 2007, 33, 125-143.], the amount of Zinc metal adsorbed per gram of adsorbent increased from 0.289 to 1.004 mg/gm as the initial concentration increased from 5 to 15 mg/l [16Folkes, M.H.; Hope, P.S. Polymer blend and alloys., 1985, , 430-440., 17Kim, J.H.; Lee, Y.M.; Kim, K.Y. Rheological study of chitosan/agar/poly vinyl alcohol blend solution. J. Appl. Polym. Sci., 1992, 44, 1823-1828.
[http://dx.doi.org/10.1002/app.1992.070441015] ]. It can be observed in Figs. (4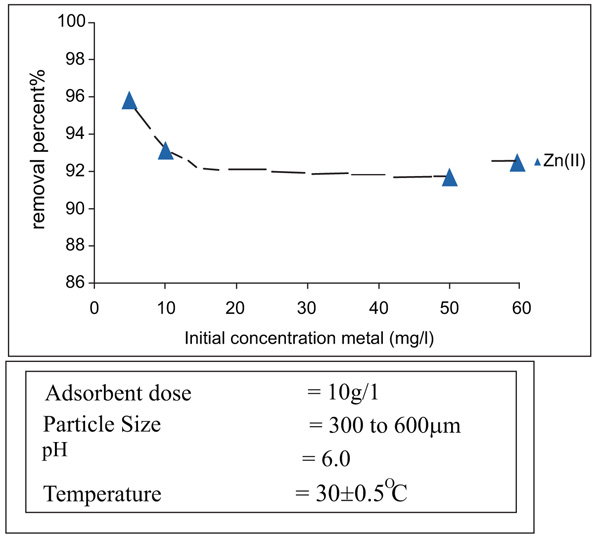 and 5
and 5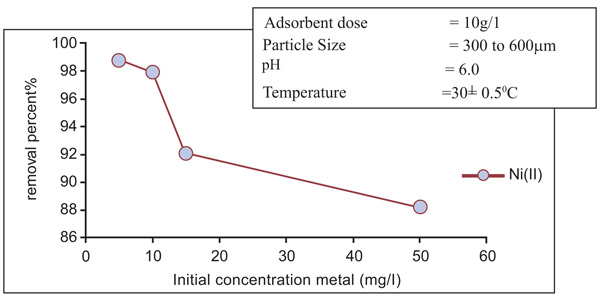 ) for nickel and Zinc that the equilibrium time is independent of initial concentration.
) for nickel and Zinc that the equilibrium time is independent of initial concentration.
 |
Fig. (4) Effect of pH on removal of Zinc(II). |
 |
Fig. (5) Effect of initial concentration on removal of Nickel (II). |
3.3. Effects of Particle Size
Increase in the specific surface area will increase the extent of adsorption process. Adsorption will be much greater for smaller particles for the specific surface area. The effects of particle size on the removal of zinc and nickel is maintained with different particle sizes of 75 to 150µm, 150- 300 µm and 350 to 600 µm. In Figs. (6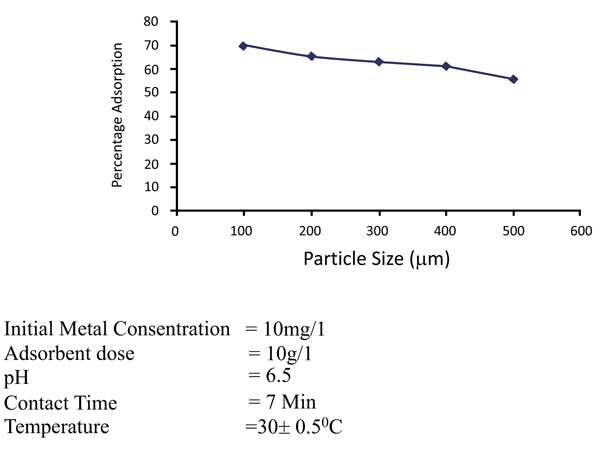 and 7
and 7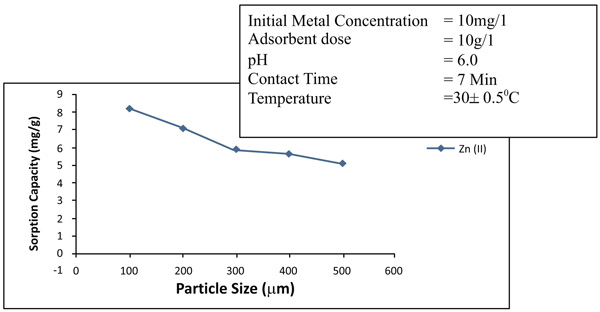 ), it has been observed that the percentage removal of zinc increased from 69.2 to 89.5% as the particle size decreased from 300- 600µm to 75 to 150 µm. In the study of nickel, the removal enhanced from 81.9 to 95.8% as the particle size lowered from 300- 600 µm to 75-150 µm Table 1.
), it has been observed that the percentage removal of zinc increased from 69.2 to 89.5% as the particle size decreased from 300- 600µm to 75 to 150 µm. In the study of nickel, the removal enhanced from 81.9 to 95.8% as the particle size lowered from 300- 600 µm to 75-150 µm Table 1.
 |
Fig. (6) Effects of particle sizes (µm) of removal of Zinc (II). |
 |
Fig. (7) Effects of particle sizes of removal of Nickel (II). |
3.4. Adsorption Isotherms
The equilibrium data for the adsorption of zinc and nickel on chitin fitted in Langmuir isotherms [18Knorr, D. Functional properties of chitin and chitosan. J. Food Sci., 1982, 47, 593-595.
[http://dx.doi.org/10.1111/j.1365-2621.1982.tb10131.x] , 19Langmuir, I. The constant and fundamental properties of solids and liquids. J. Am. Chem. Soc., 1916, 38(), 2221-2295.
[http://dx.doi.org/10.1021/ja02268a002] ] (Figs. 8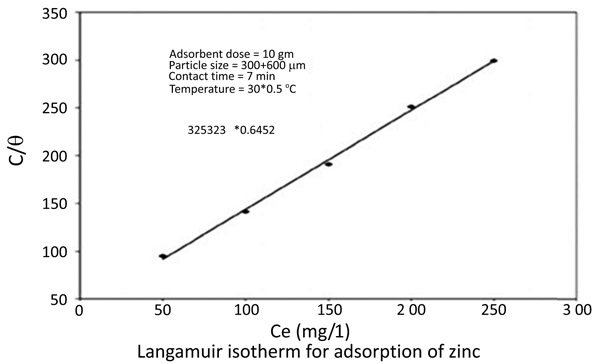 and 9
and 9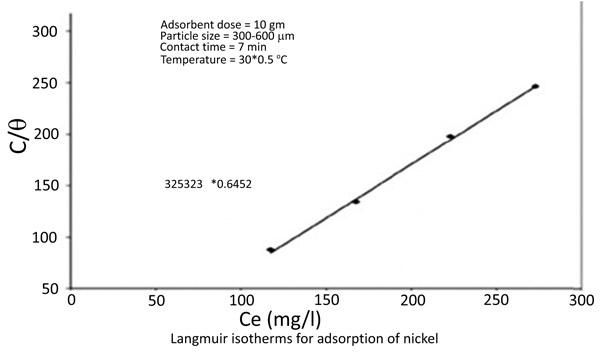 ) 1/(x/m) = 1/Qo + 1 / (bQo Ce)
) 1/(x/m) = 1/Qo + 1 / (bQo Ce)
Where
Qo – Amount of adsorbent, which form a complete monolayer on the surface (mg/gm)
b - Constant, which increases with increasing molecular size
Ce - Equilibrium concentration (mg/l) between adsorbent and solution
x - mass of gas adsorbed (mg/l)
m- grams of adsorbent (gm/l)
 |
Fig. (8) Langmuir isotherm for adsorption of Zinc. |
 |
Fig. (9) Langmuir isotherms for adsorption of Nickel. |
The Langmuir Constants are presented in the following table (Table 1)
3.5. Column Studies
The studies were conducted by downflow column by using 18mm diameter Chitin adsorbent column with a depth of 15 cm. bottom bed. The exact flow rate of 1.15 m2/hr (5ml/min) was initiated during the experiment. The breakthrough of the results is shown by the curves mentioned in Figs. (10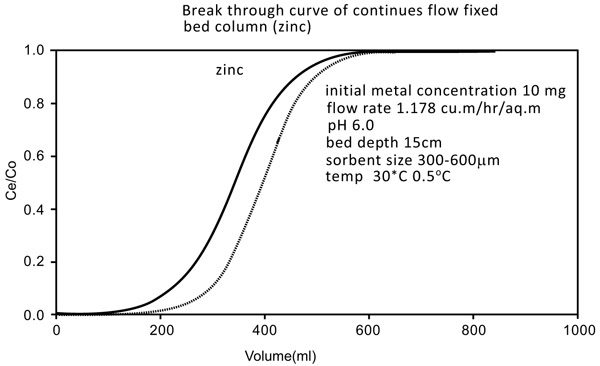 and 11
and 11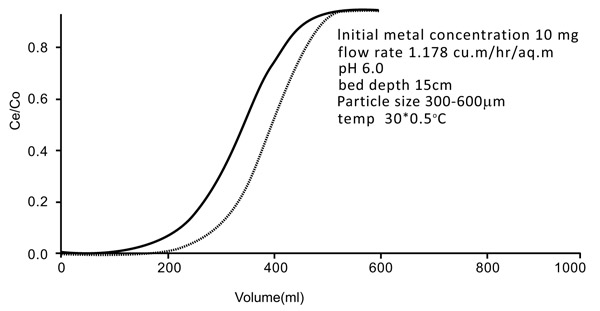 ). The breakthrough adsorption capacities and total column are mentioned below: (Table 2)
). The breakthrough adsorption capacities and total column are mentioned below: (Table 2)
 |
Fig. (10) Breakthrough curve of continue flow fixed bed column (Zinc). |
 |
Fig. (11) Breakthrough curve of continue flow fixed bed column (Nickel). |
3.6. Kinetic Rate Constants
Normally adsorption is considered to be a forward reaction of first-order kinetics (Figs. 12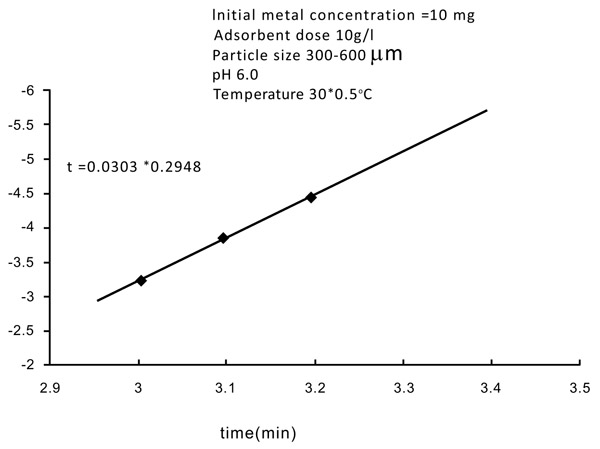 and 13
and 13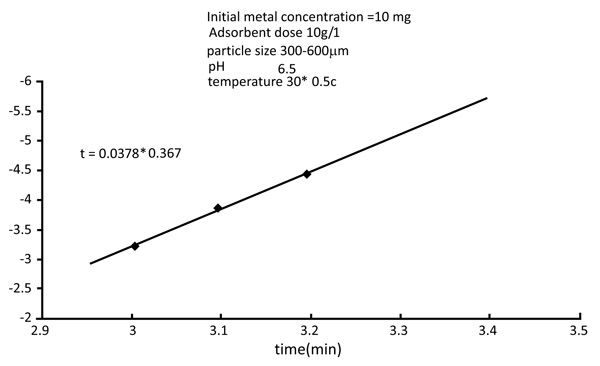 ). The kinetic rate constant provides the nature of adsorbent metal reaction. The constant rate can be determined using the following equation,
). The kinetic rate constant provides the nature of adsorbent metal reaction. The constant rate can be determined using the following equation,
Log(C/C1) = (K/2.303) t
Log (C/C1) = K1t Where
C - Initial Concentration (mg/l) of metal
C1 -Concentration remaining in solution at any time ‘t’
t -Time (min)
K1 Proportionality constant (or) kinetic rate constant
 |
Fig. (12) Kinetic rate constant of Zinc. |
 |
Fig. (13) Kinetic rate constant of Nickel. |
The rate of zinc and nickel are presented as follows in the table form (Table 3).
CONCLUSION
Chitin is a natural biopolymer which is found to be selective, more reactive and effective adsorbent for the removal of zinc and nickel from aqueous solution in comparison to other adsorbents like sawdust, clay, lime, charcoal and fly ash. The amino groups present in chitin in the coordinate covalent bonds with metal ions and is easily adsorbed. The equilibrium time for this metal using chitin is less when compared to that of other adsorbents. The removal of zinc was 71.8% and of Nickel is 85.6%.
In the beginning, the adsorption of the initial concentration of metal increased as the amount of metal adsorbed per gram of adsorbent increased. The equilibrium time in the process of adsorption is independent in the initial concentration. If the adsorbent dose is increased, the percentage removal of metal will also be increased. The percent removal of metal is also increased with the decrease in particle size of adsorbent from 300 µm to 75µm. The reaction rate of heavy metals on the Chitin follows simple first order kinetic equation.
The rate law or rate equation for chemical reaction is an equation that links the reaction rate with the concentrations or pressures of the reactants and constant parameters (normally rate coefficients and partial reaction order). For many reactions, the rate is given by a power law such as:
r = k [A] x [B] y
Where [A] and [B] express the concentration of the species A and B (usually in moles per liter (molarity, M)). The exponents x and y are the partial orders of reaction for A and B and the overall reaction order is the sum of the exponents. These are often positive integers but they may also be zero, fractional, or negative. The constant k is the reaction rate constant or rate coefficient of the reaction and has units of 1/time. Its value may depend on conditions such as temperature, ionic strength, surface area of an adsorbent or light irradiation
The kinetic constant in the study was observed as Ni (II) as higher than that of Zn (II). The adsorption of Ni (II) and Zinc (II) on the Chitin poser can be well described by Langmuir’s isotherm. Nickel shows more preference for adsorption sites in Chitin than Zinc as per the fixed bed column studies.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
| [1] | Bradl, H., Ed.; Heavy Metals in the Environment: Origin, interaction and remediation., 2002, , 6. |
| [2] | Kapoor, A.; Viraraghavan, J Fungal bio sorption an alternative option for heavy metal bearing waste water. A Review in Bio Resource Technology, 1995, 53(3), 195-206. |
| [3] | Ramya, R.; Sankar, P.; Anbalagan, S.; Sudha, P.N. Adsorption of Ni(II) from metal solution using cross linked chitosan-g-acrylonitrile copolymer. International Journal of Environmental Sciences, 2001, 1(6), 1323-1335. |
| [4] | Ravikumar, M.N.V. A review of chitin and chitosan application. React. Funct. Polym., 2000, 46, 1-27. [http://dx.doi.org/10.1016/S1381-5148(00)00038-9] |
| [5] | M, Sudha. P.; Gakwisiri, C.; Raut, N.; Al-Saadi, A.; Al-Aisri, S.; Al-Ajmi, A. A critical review of removal of zinc from wastewater Proceedings of the World Congress on Engineering, 2012, 1, 627-630. |
| [6] | Wasewar, K.L.; Mohammad, A.; Prasad, B.; Mishra, I.M. Adsorption of Zn using factory tea waste: Kinetics, equilibrium a nd thermodynamics. CLEAN: Soil. Water. Air, 2008, 36(3), 320-329. [http://dx.doi.org/10.1002/clen.200700139] |
| [7] | Crini, G.; Peindy, H.N.; Gimbert, F.; Robert, C. Removal of C.I. basic green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: Kinetic and equilibrium studies. Separ. Purif. Tech., 2007, 53, 97-110. [http://dx.doi.org/10.1016/j.seppur.2006.06.018] |
| [8] | Gupta, V.K.; Shrivastava, A.K.; Jain, N. Biosorption of Chromium(VI) from aqueous solutions by green algae Spirogyra species. Water Res., 2001, 35(17), 4079-4085. [http://dx.doi.org/10.1016/S0043-1354(01)00138-5] [PMID: 11791837] |
| [9] | Roberts, G.A.F. Chitin Chemistry., 1992, [http://dx.doi.org/10.1007/978-1-349-11545-7] |
| [10] | Koumanova, B.; Peeva, P.; Allen, S.J.; Gallgher, K.A.; Healy, M.G. Biosorption from aqueous solutions by egg shell membranes and rhizopus oryzae: Equilibrium and Kinetic studies. J. Chem. Technol. Biotechnol., 2002, 77, 539-545. [http://dx.doi.org/10.1002/jctb.601] |
| [11] | Sampranpiboon, P.; Charnkeitkong, P. Equilibrium Isotherm, hermodynamic and kinetic studies of lead adsorption onto pineapple and paper wastes. Int. J. Energy Environ., 2010, 4, 88-97. |
| [12] | Bolto, B.A. Soluble polymers in water purification. Prog. Polym Sci., 1995, 20, 987-1041. |
| [13] | a) Chiou, M.S.; Li, H.Y. Equilibrium and kinetic modeling of adsorption of reactive dye on cross-linked chitosan beads. J. Hazard. Mater., 2002, 93(2), 233-248. [http://dx.doi.org/10.1016/S0304-3894(02)00030-4] [PMID: 12117469] b) Raut, N.; Charif, G. A critical review of removal of zinc from wastewater. Proceedings of the world congress on engineering, 2012, 4-6. |
| [14] | Elangovan, R.; Philip, L.; Chandraraj, K. Biosorption of chromium species by aquatic weeds: Kinetics and mechanism studies. J. Hazard. Mater., 2008, 152(1), 100-112. [http://dx.doi.org/10.1016/j.jhazmat.2007.06.067] [PMID: 17689012] |
| [15] | Abdelwahab, O. Kinetic and isotherm studies of copper (II) removal from wastewater using various adsorbents. Egypt. J. Aquat. Res., 2007, 33, 125-143. |
| [16] | Folkes, M.H.; Hope, P.S. Polymer blend and alloys., 1985, , 430-440. |
| [17] | Kim, J.H.; Lee, Y.M.; Kim, K.Y. Rheological study of chitosan/agar/poly vinyl alcohol blend solution. J. Appl. Polym. Sci., 1992, 44, 1823-1828. [http://dx.doi.org/10.1002/app.1992.070441015] |
| [18] | Knorr, D. Functional properties of chitin and chitosan. J. Food Sci., 1982, 47, 593-595. [http://dx.doi.org/10.1111/j.1365-2621.1982.tb10131.x] |
| [19] | Langmuir, I. The constant and fundamental properties of solids and liquids. J. Am. Chem. Soc., 1916, 38(), 2221-2295. [http://dx.doi.org/10.1021/ja02268a002] |