- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Synthesis, Characterization and Biological Analysis of Some Novel Complexes of Phenyl Thiourea Derivatives with Copper
Neha Mathur1, *, Nisha Jain2, A.K. Sharma3
Abstract
Introduction:
Copper is a very important metal because all forms of life require copper metals an essential micronutrient. Various biological processes, directly or indirectly are dependent on copper metal.
Methods:
Copper soaps are used as fungicides, bacteriosides, herbicides and insecticides. Copper complexes including heterocyclic compounds have attracted our attention in a magnificent way because of its utility in catalysis and biological functions. Their mechanism of synthesis, characterization and structural insight, are crucial for comprehending the criteria of the bonding and electronic interactions between the proximate metal center and chelating atoms. But still, there is a need to explore some of more biological properties for their wide applicability and significant usage in multiple fields because it is an untapped area with potentially tremendous value. Hence, in this paper, we report the synthesis and characterization of transition metal complex of N/S ligand by chromatographic, FT-IR, NMR, ESR, elemental analysis, conductometric and magnetic moment measurements.
Results:
The synthesized metal complexes namely copper palmitate with 4-nitrophenylthiourea and copper palmitate with 4-methoxyphenylthiourea were successfully investigated for biological activities against fungi Candida albicans and Trichoderma harzinum. Based on the results, we pronounced biocidal activities of the novel complexes.
Conclusion:
It is concluded that the activity of nitro phenylthiourea complex has greater antifungal activity than methoxy phenylthiourea complex against these test fungi. We can conclude that the antifungal activity of these complexes varies according to the nature of the groups attached to the ligands.
Article Information
Identifiers and Pagination:
Year: 2018Volume: 5
First Page: 182
Last Page: 195
Publisher Id: CHEM-5-182
DOI: 10.2174/1874842201805010182
Article History:
Received Date: 28/06/2018Revision Received Date: 02/11/2018
Acceptance Date: 07/11/2018
Electronic publication date: 31/12/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Chemistry,Pt. N.K.S. Govt. P.G. College, Dausa-303303, Rajasthan, India, Tel: +91 9414738865; Email:nehavmathur@yahoo.co.in
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 28-06-2018 |
Original Manuscript | Synthesis, Characterization and Biological Analysis of Some Novel Complexes of Phenyl Thiourea Derivatives with Copper | |
1. INTRODUCTION
The present research is an important effort to understand the characteristic nature and biocidal application of copper complexes of nitrogen donor ligands. The treatment with copper complexes produces remarkable pharmacological effects which are not observed when the parent ligands or inorganic forms of copper are used [1Bhutra, R.; Sharma, R.; Sharma, A.K. Fungicidal activities of Cu (II) soaps derived from various oils treated at high temperature for biomedical use. SAJ Biotechnol, 2018, 5, 1-3., 2Sharma, A. K.; Sharma, R.; Saxena, M. Spectroscopic and Biocidal activities of environmentally safe Agrochemicals. J Biochem. Tech., 2018, 7(3), 1139-1147.]. The use of transition metal complexes as therapeutic compounds has become more and more pronounced. The chemistry of substituted thiourea derivatives has attracted attention because of their potential use as reagents for the separation of metal ions and in medicinal chemistry. Thioureas are versatile ligands, able to co-ordinate to metal centers either as neutral ligands, monoanions or dianions. O, N and S donor atoms of thiourea derivatives provide a multitude of bonding possibilities [3Kapoor, P.; Fahmi, N.; Singh, R.V. Microwave assisted synthesis, spectroscopic, electrochemical and DNA cleavage studies of lanthanide(III) complexes with coumarin based imines. Spectrochim. Acta A Mol. Biomol. Spectrosc., 2011, 83(1), 74-81.
[http://dx.doi.org/10.1016/j.saa.2011.07.054] [PMID: 21903455] , 4Mathur, N.; Dobhal, M.P.; Jain, N. A comparative antibacterial analysis of substituted benzothiazole and substituted phenylthiourea complexes with copper metal. Int. J. Green Herb. Chem., 2017, 7(1), 1-6.]. The role of benzoyl thiourea derivatives in co-ordination chemistry has been extensively studied and quite satisfactorily elucidated. New thiourea derivatives and their structures have received attention because of their wonderful complexation capacity [5Raj, B.K.; Kumar, A. Synthesis, characterization and biocidal activity of some Schiff base and its metal complexes of Co (II), Cu (II) and Ni (II). Orient. J. Chem., 2013, 29(3), 1187-1191.
[http://dx.doi.org/10.13005/ojc/290349] ]. Various physical properties i.e. density, viscosity, fluidity of the complexes in non -aqueous medium (Propanol + Benzene) have been investigated to determine critical micelle concentration [6Tank, P.; Sharma, A.K.; Sharma, R. Thermal behaviour and kinetics of copper (II) soaps and complexes derived from mustard and soyabeanOil. J. Anal. Pharm. Res., 2017, 4(2), 1-5.
[http://dx.doi.org/10.15406/japlr.2017.04.00102] ]. These physical parameters are used to understand molecular interactions between the components of the mixture [7Mathur, N.; Bargotya, S. Evaluation of novel N/S containing heterocyclic metal complexes as biologically potent agents. Res. J. Chem. Sci., 2018, 8(4), 30-34.]. Information is also provided about solute–solute and solute-solvent interactions and structural insight of micelles. Present work generates new hopes in the pharmacological field [8Bargotya, S.; Mathur, N.; Sharma, A.K. Antimicrobial & DNA cleavage studies of heterocyclic metal complexes“ISBN978-613-8-34627-2., 2018, ]. Owing to the aforesaid applications of copper and heterocyclic compounds, we have synthesized copper complexes containing heterocyclic ligands. Out of them, various copper complexes have been reported to inhibit bacterial, fungal, yeast, algal, mycoplasma and viral growth as well as are to cause the death of these microorganisms [9Sharma, S.; Sharma, R.; Sharma, A.K. Synthesis, characterization, and thermal degradation of Cu (II) surfactants for sustainable green chem. Asian J. Green Chem., 2017, 2(2), 129-140.
[http://dx.doi.org/10.22631/ajgc.2017.95559.1015] , 10Sharma, A.K.; Sharma, S.; Sharma, R. Thermal degradation of Cu (II) metallic Soaps and their Characterizations. A Pharmaceutical Application Chron. Pharm. Sci., 2017, 1(5), 312-319.]. Copper complexes are also used as antitumor agents [11Mishra, A.P.; Mishra, R.K.; Shrivastava, S.P. Structural and antimicrobial studies of coordination compounds of VO(II), Co(II), Ni(II) and Cu(II) with some Schiff bases involving 2-amino-4-chlorophenol. J. Serb. Chem. Soc., 2009, 74, 523-535.
[http://dx.doi.org/10.2298/JSC0905523M] ], anti-inflammatory [12Pillai, V.V.; Sreekanth, B. DNA binding and antimicrobial studies of Ag (II) and Cu (II) metalcomplexes containing mixed ligands of 1, 10- phenanthroline and 8-hydroxyuinoline. Int. J. Pharma. Bio. Sci., 2013, 4, 739-747.], enzyme inhibitors or chemical nuclease [13Jain, P.; Kachhwaha, S.; Kothari, S.L. Chloroplast ultra structure, photosynthesis and enzyme activities in regenerated plants of Stevia rebaudiana (Bert.) Bertoni as influenced by copper sulphate in the medium. Indian J. Exp. Biol., 2014, 52(9), 898-904.
[PMID: 25241590] ]. The study and development of copper complexes could be helpful in the design and production of antiviral and antifungal materials. It is also able to deactivate HIV viruses and antibiotic-resistant bacteria [14Mathur, N.; Bargotya, S.; Manna, B.; Kasana, A. Antifungal screening and oxidation behaviour of some microwave assisted benzothaizine drugs. Int. J. Chem. Sci., 2014, 12(2), 533-546.]. In the present study antifungal activity was performed for the synthesized complexes CP(PTU)NA, CP(PTU)A, on Muller Hinton Agar against Candida albicans, Trichoderma harzianum Abbreviation of complexes as follows:
CP[PTU]NA: Complex of Cu (II) Palmitate with 4-nitrophenylthiourea
CP[PTU]A: Complex of Cu (II) Palmitate with 4-methoxyphenylthiourea
2. EXPERIMENTAL
2.1. Preparation of Substituted Phenylthiourea
In this method, Arylamine was treated with ammonium thiocyanate to prepare phenylthiourea. For this purpose, 13.8 g/12.3 g (0.1 mol) p-nitroaniline / p-methoxy aniline was heated in a 250 ml three-necked flask with stirrer, dropping funnel and reflux condenser with a mixture of 9 ml (6N HCl) and 25 ml water at a temperature of 32°C on water bath till the aniline hydrochloride was formed. The solution now obtained is allowed to cool at room temperature and then 7.6 g (1 mol) ammonium thiocyanate was added to it. The reaction mixture was refluxed for about four hours on a water bath. After cooling the solid separated out was filtered, washed with cold water, dried and then recrystallized with ethanol (Schemes 1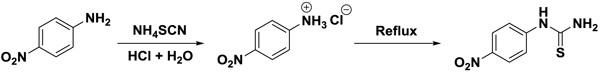 and 2
and 2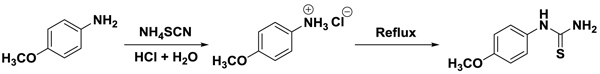 ).
).
 |
Scheme 1 Synthesis of phenylthiourea of from 4-nitro aniline. |
 |
Scheme 2 Synthesis of phenylthiourea of from 4-methoxy aniline. |
2.2. Preparation of Copper Palmitate Soap
Copper palmitate was prepared by mixing 1g of palmitic acid into 25 ml ethyl alcohol, shake the mixture in hot water bath at about 50°C and then add one drop of phenolphthalein. Prepare a saturated solution of KOH in another beaker and add it drop by drop into the first beaker until the light pink color appears. Meanwhile, in another beaker prepare a saturated solution of CuSO4 (approx 3-4 g in 5 ml H2O) and mix it into the above solution with constant stirring till the blue coloured soap is formed. Filtered it and washed with warm water and 10% ethyl alcohol, then dried and recrystallized with hot benzene (Scheme 3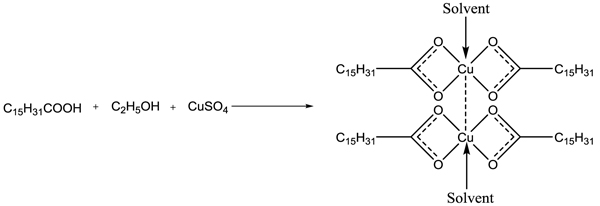 ).
).
 |
Scheme 3 Synthesis of copper palmitate soap. |
2.3. Preparation of Complex Using Soap and Ligand
Complexation of purified copper palmitate obtained from palmitic acid and substituted phenylthiourea was done by adding 0.001-mole copper palmitate with 0.002-mole phenylthiourea in 25-30 ml ethyl alcohol and the mixture was refluxed for about two hours with constant stirring (Schemes 4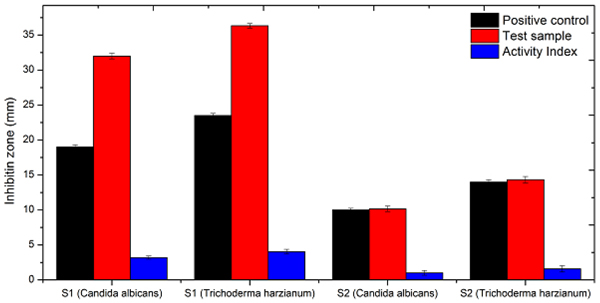 and 5
and 5 ). After cooling, the solid separated out was filtered, dried and recrystallized with hot benzene.
). After cooling, the solid separated out was filtered, dried and recrystallized with hot benzene.
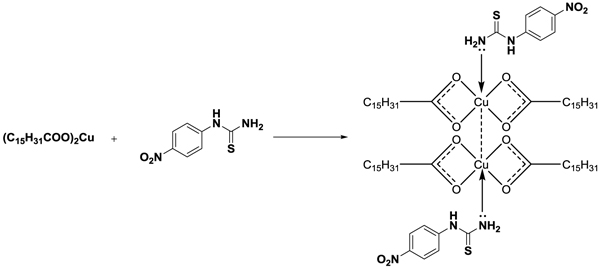 |
Scheme 4 Synthesis of complex from copper palmitate soap and 4-nitro phenyl thiourea. |
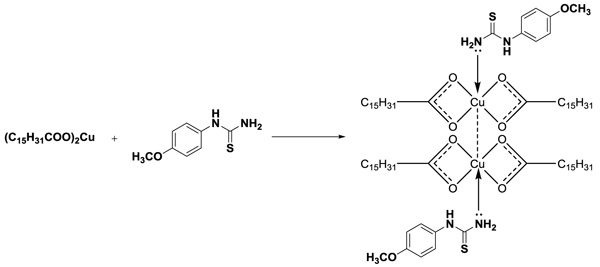 |
Scheme 5 Synthesis of complex from copper palmitate soap and 4-methoxy phenyl thiourea. |
3. CHARACTERIZATION
The analytical and physical data of ligand, soap and complexes are summarized in Table 1. The synthesized complexes characterized by chromatography and spectroscopic techniques as follows:
3.1. Chromatography
TLC is probably the most versatile technique for the analysis of complexes and their mixtures. TLC is quite an effective technique for separation and identification of synthesized soap, ligand and their complexes in sub-microgram amounts using silica gel plates in various non-aqueous solvent systems. Rf of copper soap, ligands and the synthesized complexes in non-aqueous solvent systems has been determined. A thin layer chromatographic applicator was used for coating sorbent material on 20×3 cm glass plates to obtain an absorbent layer of 0.25 mm thickness [15Sharma, A.K.; Sharma, R.; Saxena, M. Synthesis, spectroscopic and fungicidal studies of Cu (II) soaps derived from groundnut and sesame oils and their urea complexes. Bulletin Pure Appl. Sci., 2017, 36(2), 26-37.
[http://dx.doi.org/10.5958/2320-320X.2017.00004.8] ]. The coated plates were developed in glass jars of 24×6 cm. A glass sprayer was used to spray chromogenic reagent on the plate to detect the spot. Silica gel G acted as stationary phase whereas the following solvent systems were used as mobile phase.
- Acetone: Carbon tetrachloride
- Acetone: Petroleum ether
- Benzene: Acetone: Petroleum ether
Silica gel G and double distilled water are mixed in 1:3 (w/v) ratio with constant mechanical shaking until the homogenous slurry was obtained to prepare the plates. Approx. 10μl of the test solution was applied on thin layer plates by using micropipette. The solvent ascent was fixed to 10-12 cm in all cases. When the development was completed, the plates are withdrawn from the jar and dried at room temperature. The spots were visible in daylight.
3.2. Spectroscopy
The spectral measurements (IR and NMR) and elemental analysis were carried out at the (RSIC, CDRI) Lucknow U.P. India. The IR spectra of the complexes were obtained as KBr discs in the range 400-4000 cm–1 on Perkin Elmer spectrophotometer. 1H NMR spectra were recorded at CDRI, Lucknow using CDCl3 as a reference. ESR spectra of the complexes were recorded at SAIF, IIT, Mumbai in the X-band region at microwave frequency 9.1 GHz and microwave power 5 mW under the magnetic field of strength 2000G at room temperature. (TCNE) was used as the standard. Magnetic susceptibility measurements were conducted at S.P. University, Vallabh Vidyanagar, Gujrat.
3.3. Biocidal
Step 1: Processing of the Sample.
The unknown compound is suspended in DMSO in mass concentration (w/v).
Step 2: Antimicrobial Susceptibility Testing (Kirby-Bauer and Stokes' methods.) [16Mathur, N.; Bargotya, S. Advances in the Research of Novel N/S Containing Metal Complexes as Potent Antioxidants. J. Appl. Chem., 2017, 10(8), 18-23.].
I. Hinton Susceptibility Test Agar
Sabouraud Dextrose Agar is the only susceptibility test medium that has been validated by NCCLS. Mueller-Hinton agar should always be used for disk diffusion susceptibility testing, according to Fig. (1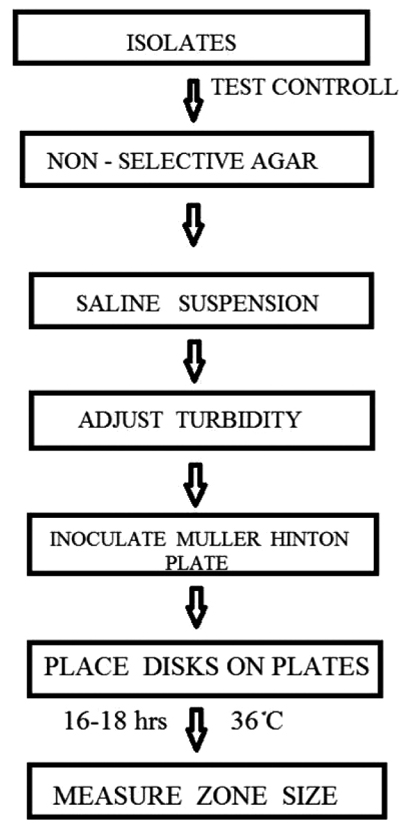 )
)
II. McFarland turbidity standard
A McFarland 0.5 standard should be prepared and quality controlled prior to beginning susceptibility testing. If tightly sealed to prevent evaporation and stored in the dark, the standard can be stored for up to 6 months. The McFarland standard is used to adjust the turbidity of the inoculum for the susceptibility test
III. Preparation of inoculum
Each culture to be tested should be streaked onto a no inhibitory agar medium to obtain isolated colonies. After incubation at 35°C overnight, select 4 or 5 well-isolated colonies with an inoculating needle or loop and transfer the growth to a tube of sterile saline or nonselective broth (Mueller-Hinton broth, Peptone water) and vortex thoroughly.
IV. Inoculation procedure
a. Within 15 minutes after adjusting the turbidity of the inoculum suspension, dip a sterile cotton swab into the suspension. Pressing firmly against the inside wall of the tube just above the fluid level, rotate the swab to remove excess liquid. Streak the swab over the entire surface of the medium three times, rotating the plate approximately 60 degrees after each application to ensure an even distribution of the inoculum. Finally, swab all around the edge of the agar surface.
b. The Mueller-Hinton plate should be swabbed over the entire surface of the medium three times, rotating the plate 60 degrees after each application.
V. Loading the plate with Positive, Negative Control and Sample
a. The working supply of antibiotic (streptomycin, positive control) should be stored in the refrigerator (4°C). 50 µl of the antibiotic suspension was dispensed in the well labeled with C (control) to the plates as soon as possible, but no longer than 15 minutes after inoculation. Diffusion of the drug in the well begins immediately.
b. 50 µl of the sample (S) and 50 µl of the reference (R, negative control) were dispensed in the well labeled with C (control) to the plates as soon as possible, but no longer than 15 minutes after inoculation.
VI. Recording and interpreting results
After the Loading of C, S, R on the plate, invert the plate and incubate at 35°C for 16 to 18 hours. After incubation, measure the diameter of the zones of complete inhibition (including the diameter of the well) and record it in millimeters. The measurements can be made with a ruler on the undersurface of the plate without opening the lid. The zones of growth inhibition should be compared and recorded as susceptible, intermediate, or resistant to each drug tested. Colonies growing within the clear zone of inhibition may represent resistant variants or a mixed inoculum. The distance from the colonies closest to the well to the center of the well should be measured and then doubled to obtain a diameter. The diameter of the outer clear zone should be recorded as well and an interpretation recorded for each diameter. The colonies inside the zone should be picked, re-isolated, re-identified, and retested in the well diffusion test to confirm the previous results. The presence of colonies within a zone of inhibition may predict eventual resistance to that agent.
Note:
• Dimethyl sulfoxide (negative control) does not show any activity against the test organism.
• Antifungal Sensitivity (Itraconozole 5 mg/well) serves as a positive control.
• Sample (unknown chemical compound) (5mg/ well (w/v))
 |
Fig. (1) Flow diagram for antimicrobial study. |
4. RESULTS AND DISCUSSION
4.1. Chromatography
The results were reproducible with in ±0.02 Rf unit values for various substituted compounds using the procedure for three solvent systems – the main part of the chromatographic studies was an attempt to obtain the standard conditions for their resolution and identification. The best shaped spots given by the solvents were considered to be suitable, and are A = Acetone = carbon tetrachloride, B = Acetone = Petroleum ether, C = Benzene = Acetone = Petroleum ether. In pure acetone spots run along with the solvent front hence could not be used [17Sharma, A. K.; Sharma, R.; Saxena, M. Biomedical and antifungal application of Cu(II) soaps and its urea complexes derived from various oils. Open Access J. Trans. Med. Res., 2018, 2(2), 40-43.]. The single spot was given by all compounds, uniform in shape in the present investigation, so all of them are chromatographically pure. The results of copper palmitate, substituted phenylthiourea and complexes are given in Table 2 [18Bargotya, S.; Mathur, N.; Sharma, A.K. Physico-chemical analysis of environmentally safe complexes of copper “ISBN978-613-8-34672-2”., 2018, ].
4.2. Spectroscopy
(1). IR Spectral studies:
The IR spectra of the complexes with relative intensities/peaks are given in Table 3.
(A). Cu (II) Palmitate:
The IR spectrum of copper palmitate asymmetric stretching vibration of methyl and methylene group is observed at 2910 cm–1 whereas, the symmetric stretching vibration of the group occurred at lower frequency i.e. at 2840 cm–1. The disappearance of the characteristic bands of esters and appearance of the bands of carboxylate ion indicate the formation of copper palmitate soap. The absence of C=O band in the spectra of soap indicate that there is a resonance in the two C=O bonds of carboxylate group [19Mathur, N.; Bargotya, S. DNA–Binding and cleavage studies of macrocyclic metal complexes containing heteroatomic ligands. Chem. Sci. Trans., 2016, 5(1), 117-124.]. On comparing the IR spectra of palmitic acid and copper palmitate, it is found that the acid (palmitic acid) exists with dimeric structure through hydrogen bonding between carboxyl group of the two acid molecules whereas, copper palmitate soap is ionic in nature and the metal to oxygen bond has ionic character. The band in the region 485 cm–1 is due to copper to oxygen bond stretching vibration. This is called characteristic absorption of metal constituent of soap molecule [20Bhutra, R.; Sharma, R.; Sharma, A.K. Synthesis, Characterization and fungicidal activities of Cu (II) surfactants derived from groundnut and mustard oils treated at high temperatures. J. Inst. Chemists (India), 2018, 90(3), 66-80.].
(B). CP[PTU]A: Complex of Cu (II) palmitate with 4-methoxy phenylthiourea
The detailed infrared spectral investigation reveals that there are marked differences between the spectra of copper palmitate (soap) and free ligands than those of corresponding complexes.
The absorption bands observed in the region 2920 cm–1 and 2848 cm–1 correspond to C-H symmetric and asymmetric stretching of methyl (-CH3) and methylene (-CH2) groups of the soap segment present in the complex. The band resulting from the methylene rocking vibrations, in which all of the methylene groups rocks in phase, appears near 725 cm–1 whereas absorption, due to methyl rocking vibrations is observed in the 1110 cm–1 region [21Mathur, N.; Sharma, A.K. Synthesis of Hetero-cyclic compounds for Bio-medical Applications., 2018, ]. Absorption due to –CH2 twisting and wagging vibration is observed at 1360 cm–1. The carboxylate ion gives rise to two bands i.e. a weak asymmetrical stretching band near 1510.1 cm–1 and another symmetrical stretching band at 1470 cm–1. The band in the region 505.6 cm–1 is due to vM-O symmetric stretching. This is called characteristic absorption of metal constituent of soap molecule.
The thiocarbonyl moiety of the ligand shows absorption in the 1170 cm–1 region. The complex contains C=S group which is attached to a nitrogen atom, shows an absorption band in the general C=S stretching region. In addition, several other bands in the broad region of 1510 cm–1 can be attributed to vibrations involving interactions between C=S stretching and C-N stretching. Complex has N-C=S group which is an essential structural feature responsible for various biocidal properties. N-C=S structure band occurs near 1310 cm–1. (Fig. 2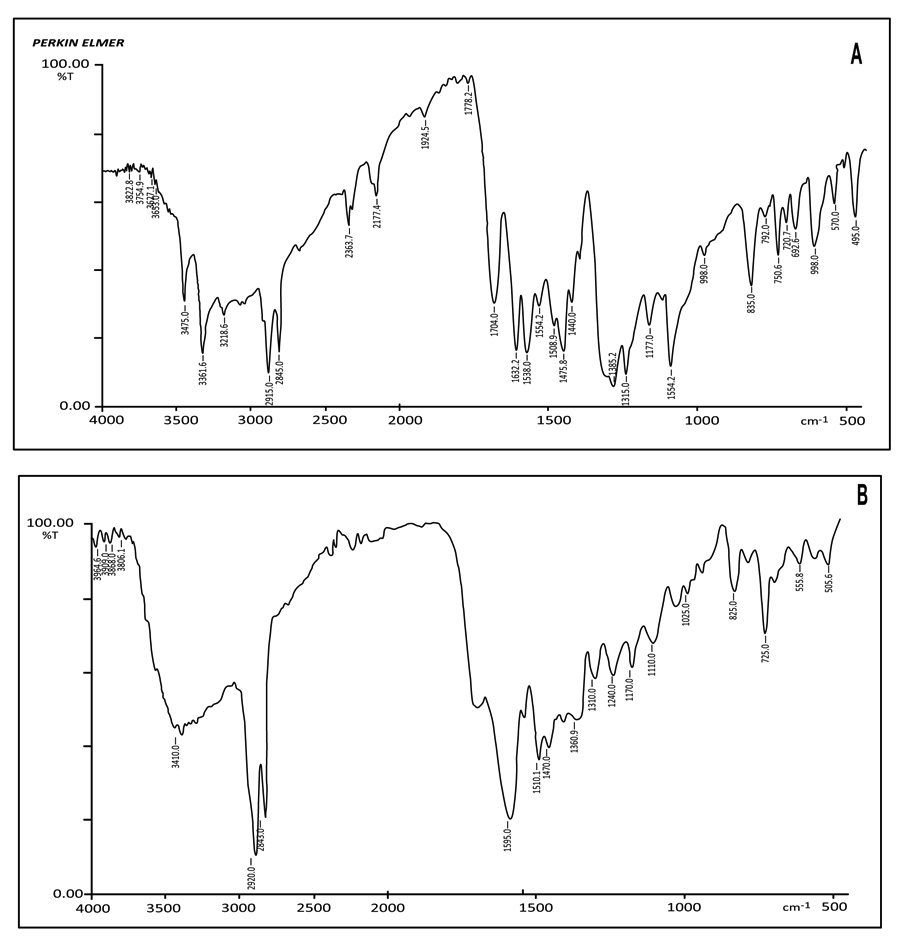 )
)
 |
Fig. (2) IR Spectra of Complex: A: Copper (II) Palmitate with 4-Methoxy Phenylthiourea – CP[PTU]A B: Copper (II) Palmitate with 4-Nitro phenylthiourea – CP[PTU]NA |
The most prominent bands in the spectra of aromatic compounds occur in the low-frequency range between 900 cm–1 and 670 cm–1. These strong absorption bands result from the out of plane (OOP) bending of the ring C-H bonds, which is found in this complex at 825 cm–1. The N-H stretching vibration appeared near 3410 cm–1. The spectra of phenylthiourea complex contain Ar-O-CH3 group, which display an `asymmetrical stretching vibration at 1240 cm–1 and symmetrical stretching vibration near 1025 cm–1.
The strong band at 1615 cm–1 is a characteristic feature of the N-H bending vibration of the NH2 group in the free ligand (4-methoxy phenylthiourea) but in this complex, it is shifted to lower frequency at 1595 indicating that the primary nitrogen is the coordinating site in this complex. The conclusive evidence of bonding of metal to ligand is indicated the appearance of band (vM-N) at 555.8 cm–1. These values are comparable with other reported complexes [22Joram, A.; Sharma, R.; Sharma, A.K. Thermal degradation of complexes derived from Cu (II) groundnut soap (Arachis hypogaea) and Cu (II) sesame soap (Sesamum indicum). Z. Phys. Chem., 2018, 232(4), 459-470.
[http://dx.doi.org/10.1515/zpch-2017-1073] ].
(C). CP[PTU]NA: Complex of Cu (II) palmitate with 4-nitro phenylthiourea
The asymmetric stretching vibration of methyl and methylene group is observed at 2915 cm–1 whereas the symmetric stretching vibration of the above group is observed at lower frequency 2845 cm–1. Two bands resulting from the methyl and methylene rocking vibrations appeared at 1110.8 cm–1 and 720.7 cm–1 region. The band appearing at 1395 cm–1 is due to methylene twisting and wagging vibrations. The spectrum shows two bands at 1508.9cm–1 and 1475.8 cm–1 attributed, respectively to v(C-O)antisymmetric and v(C-O) symmetric vibrations of carboxylate group. The bond in the region 495.0 cm–1 is due to v(Cu-O).
Apart from these bands, the following bands were also found corresponding to ligand moiety. In case of CP[PTU]NA, a clear peak is observed at 1440 cm–1 corresponding to Ar-C-NO2 vibrations. The N-H stretching vibration region occurs at 3475 cm–1. The band near 1315 cm–1 may be due to the N-C=S symmetric vibration. The band appearing at 1177 cm–1 is due to v(C=S) stretching while other band at 835 is due to the v(C-H) deformation [23Sharma, A.K.; Sharma, R.; Gangwal, A. Antifungal activities and characterization of some new environmentally safe Cu (II) surfactants substituted 2-amino-6-methyl benzothiazole. Open Phar. Sci. J., 2018, 5, 3-12.].
The strong band at 1602 cm–1 is a characteristic feature of the N-H bending vibration of the -NH2 group in the free ligand (4-nitro Phenylthiourea) but in this complex, it is shifted to lower frequency at 1595 cm–1 indicating that the primary nitrogen is the coordinating site in this complex. The new band appeared in the region 570 cm–1which was absent in ligand may be due to formation of Cu-N bond. The above arguments indicate that the ligand behaves as monodentate and is coordinating with copper metal through a primary nitrogen atom of NH2 group.
(2). NMR Spectral studies:
The NMR parameters are given in Table 4.
(A). CP[PTU]A: Complex of Cu (II) palmitate with 4-methoxy phenylthiourea
The ligand to metal bonding is further supported by 1H NMR spectra. The complex showed broadened peak at δ 3.70-3.78 ranges due to the protons. This peak indicates the co-ordination through the –NH2 group of phenylthiourea segment to the metal (copper) atom of the soap segment. In addition to the above signal, resonances corresponding to CH3 and CH2 protons attached to -CH2-R groups are also observed in δ 0.88 and δ 1.20region.
(B). CP[PTU]NA: Complex of Cu (II) palmitate with 4-nitro phenylthiourea
The ligand to metal bonding is further supported by 1H NMR spectra. The complex snowed broadened peak at δ 3.65-3.70 ranges due to the protons. This peak indicates the co-ordination through the –NH2 group of phenylthiourea to the metal (copper) atom of the soap segment. In addition to the above signal, resonance corresponding to CH3 and CH2 protons attached to –CH2-R groups is also observed in δ 0.90 and δ 1.22 region.
(3). ESR Spectral studies:
ESR spectrum is characterized by the position, intensity and shape of the component lines. The position of ESR is referred to as g-values and is directly determined by the energy levels. The variation in g-value is interpreted in terms of first and second order spin-orbit interaction. The g-value for ESR signal is calculated by the formula [24Khan, S.; Sharma, R.; Sharma, A.K. Antifungal Activities of Copper Surfactants derived from Neem (AzadirectaIndica) and Karanj (Pongamiapinnata) Oils: A Pharmaceutical Application. Glob. J. Pharmaceu. Sci., 2017, 3(4), 1-6.]. The ESR parameters are given in Table 5.
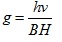 |
Where
v = Frequency of band in KHz
B = Bohr Magneton
H = Magnetic field
h = Plank's constant
On the basis of g|| and g| the values of gav and G were calculated using the following relations [25Mathur, N.; Heda, L.C.; Mathur, V.K.; Saxena, P. Study of CLSI-M44-A Disk Diffusion method for determining the susceptibility of candida species against novel complexes derived from copper stearate with 2-amino benzothiazoles. Tenside Surf. Det., 2011, 48(1), 1-5.
[http://dx.doi.org/10.3139/113.110099] ].
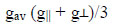 |
(A). CP[PTU]A: Complex of Cu (II) palmitate with 4-methoxy phenylthiourea
The ESR spectrum of Cu(II) complex is recorded at room temperature. The g-tensor value of the copper complex can be used to derive the ground state. In octahedral complexes, the unpaired electron may lie in a thedx2-y2or dz2 orbital. The Spin-Hamiltonian parameters for the copper complex are calculated from the spectra are 2.35, 2.05, 2.15 etc. g-tensor values are g||> g┴> g0 suggest that complex has distorted octahedral geometry with unpaired electron lying in dx2-y2 orbital.
The ESR parameters of the complex coincide well with the related systems, which suggest that the complex has octahedral geometry and the systems are axially symmetric. In the axial spectra, the g-values are related to exchange interaction coupling constant (G) by the expression [26Sharma, R.; Sharma, A.K. Natural edible oils: Comparative health aspects of sesame, coconut, mustard (Rape Seed) and Groundnut (Peanut) A Biomedical Approach. Biomed. J. Sci. Tech. Res., 2017, 1(5), 1-4.].
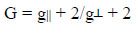 |
According to Hathaway, if the value is larger than four, the exchange interaction is negligible because the local tetragonal axes are aligned parallel or slightly misaligned. If the value is less than four, the exchange interaction is considerable and the local tetragonal axes are misaligned. For the present copper complex, it is higher than 4, which misaligned suggests that the local tetragonal axes are aligned parallel or slightly misaligned and consistent with a dx2-y2 ground state.
(B). CP[PTU]NA: Complex of Cu(II) palmitate with 4-nitro phenylthiourea
The value of g||, g┴ and gav calculated is 2.37, 2.08, 2.012 and 4.122, respectively. The g tensor values are g||> g┴> g0. The order of g values obtained from ESR spectra indicated the presence of an octahedral environment around the copper (II) ions and a distortion from regular octahedron has taken place in the shape of the complex. It suggests that the unpaired electron lies predominantly in the dx2-y2 orbital of Cu (II) giving 2B1g as the ground state. Since the value of G is greater than four for a complex of copper palmitate with 4-nitro phenylthiourea; it is suggestive of negligible exchange coupling interaction between the two copper centers in complexes.
4.3. Magnetic Moment Studies
As is reported earlier, the presence of copper ion leads to a magnetic moment of 1.73 BM. Various scientists have reported subnormal values for the magnetic moment of copper soap and fatty acid, inferring that the soap exists as a binuclear molecule. This has been attributed to the formation of δ-bonds arising from inter-molecular exchange demagnetization through the lateral overlapping of 3dx2-y2 orbital on each copper ion. The studies also reveal that these values of magnetic moments in the range 1.92-2.08 BM is due to the sub-dispersion unit of soap/complex which retains its binuclear configuration even in solution. It may be suggested that sub-dispersion units are bound together by weak polar forces resulting from the interaction between 3dxy and 3dxz orbital of two copper atoms. The lower values of magnetic moments of copper ions in copper complex in benzene, as compared to those in solid state, reflect the terminals of binuclear molecules as [Cu2(RCOO)4L2] Where L represents the solvent molecule in solution but is replaced by the ligand on complexation [27Mathur, N.; bargotya, S.; Mathur, R. Thermogravimetric analysis of microwave assisted novel macromolecular complexes of metal surfactants. J. App. Chem., 2014, 3(2), 712-719.].
4.4. Conductivity Studies
The nature of the complexes is found to be non-electrolytic as the molar conductance values in benzene, lies in the range of 3.9-6.14 mhos cm2mol-1 [28Tank, P.; Sharma, R.; Sharma, A.K. A Pharmaceutical approach & Antifungal activities of Copper Soaps with their N & S donor complexes derived from Mustard and Soyabean oils. Glob. J. Pharmaceu Sci., 2017, 3(4), 1-6.]
4.5. Biocidal Studies
The study aims to evaluate the bioactivity of synthesized complexes against test organisms (Fig. 3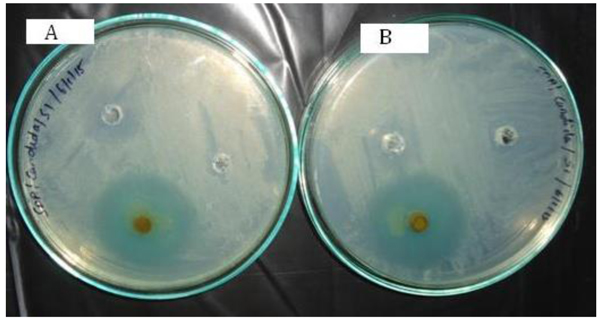 ). Antimicrobial sensitivity was performed for synthesized complexes (Compound S1, S2) on Muller Hinton Agar against Candida abicans, Trichoderma harzianum by Kirby-Bauer Stokes' method [29Mathur, N.; Manna, B. TGA analysis of transition metal complexes derived from phenothiazine ligands. Int. J. Sci. Eng. Res., 2018, 6(3), 47-57.]. Results obtained using Itraconazole (5 mg (w/v)) as antifungal agent control (C) and for negative reference, Ethanol/Methanol (R) are mentioned in Table 6 and Fig. (4
). Antimicrobial sensitivity was performed for synthesized complexes (Compound S1, S2) on Muller Hinton Agar against Candida abicans, Trichoderma harzianum by Kirby-Bauer Stokes' method [29Mathur, N.; Manna, B. TGA analysis of transition metal complexes derived from phenothiazine ligands. Int. J. Sci. Eng. Res., 2018, 6(3), 47-57.]. Results obtained using Itraconazole (5 mg (w/v)) as antifungal agent control (C) and for negative reference, Ethanol/Methanol (R) are mentioned in Table 6 and Fig. (4 )
)
 |
Fig. (4) Plots presenting the comparative study of sensitivity of fungi against complexes. |
Activity index = zone of inhibition of sample[S]/zone of inhibition of reference [R] and the activity can be found out by
Activity = Activity index x 10
If the Zone of Inhibition is [30Sharma, A.K.; Sharma, R.; Gangawal, A. Biomedical and fungicidal application of copper surfactants derived from pure fatty acid. Org. Med. Chem. I J., 2018, 5(5), 1-3 .]
< 13 = it means that the extract is inactive,
13 – 18 = it means that the extract is bioactive,
> 18 = it means that the extract is highly active
The results of ANOVA [31Mathur, N.; Bargotya, S. A facile synthesis and biological evaluation of some macro cyclic copper complexes. Int. J. Pharm. Sci. Res., 2015, 6(6), 2538-2345.] for the antifungal activities for all complexes are shown in Table 7. The predicted R2 are in reasonable agreement and closer to1.0. This confirms that the experimental data are well satisfied. The descriptive statics results of all complexes also confirm satisfactory results in triplet [32Mathur, N.; Bargotya, S. Synthesis characterization and plant growth regulatory activity of metal complexes with some bioactive ligands. World J. Pharm. Pharm. Sci., 2016, 5(11), 945-955.]. This work also coincided with Sharma et al. [33Sharma, A.K.; Saxena, M.; Sharma, R. Fungicidal activities and characterization of novel biodegradable Cu (II) surfactants derived from lauric acid. Open Chem. J., 2018, 5, 89-101.
[http://dx.doi.org/10.2174/1874842201805010089] ], Mathur et al. [34Mathur, N.; Jain, N.; Sharma, A.K. Biocidal activities of substituted benzothiazole of copper surfactants over Candida albicans & Trichoderma harzianumon Muller Hinton Agar. Open Pharm. Sci. J., 2018, 5, 24-35.
[http://dx.doi.org/10.2174/1874844901805010024] ], Bhutra el al. [35Bhutra, R.; Sharma, R.; Sharma, A.K. Antimicrobial studies and characterization of copper surfactants derived from various oils treated at high temperatures by P.D.A. technique. Open Phar Sci. J., 2018, 5, 36-40.
[http://dx.doi.org/10.2174/1874844901805010036] ] on fungicidal activities of copper surfactants derived from lauric acid, substituted complex of benzothiazole of copper palmitic acid and copper surfactants derived from fried oils treated and untreated at higher temperatures, respectively.
CONCLUSION
The newly synthesized copper (II) complexes have been tested on various biocidal field’s namely antifungal activities. This complexation can be used for innovating promising prospective antibiotic agents against some known pathogenic organism and can be used as marketed drugs. It is confirmed that the activity of complex of Cu (II) palmitate with 4-nitro phenylthiourea (S1) against Candida albicans and Trichoderma harzinum is greater than the activity of complex of copper palmitate with 4-methoxy phenylthiourea (S2) against these test fungi. We can conclude that antifungal activity of various complexes of copper palmitate varies according to the nature of the groups attached to the ligands which attached to soap. Copper complexes are recommended as therapeutic compounds. In the present study, the synthesized complexes are checked to inhibit the growth of various fungi and bacteria. The complexes explored have a wide array of applications. Such complexes are useful in the paint industry to avoid any fungal growth, in the textile industry to avoid infections and in many touches, surfaces to reduce the spread of infectious microorganisms. These complexes can be used in polymer industries efficiently.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors express their sincere thanks to the Principal, Head of the Department of Chemistry, Pt. N.K.S. Govt. P.G. College, Dausa for providing laboratory facilities. Heart full thanks to Therachem laboratories, Jaipur for NMR spectral data’s, IIT Mumbai for FT-IR and ESR spectral data’s.
REFERENCES
| [1] | Bhutra, R.; Sharma, R.; Sharma, A.K. Fungicidal activities of Cu (II) soaps derived from various oils treated at high temperature for biomedical use. SAJ Biotechnol, 2018, 5, 1-3. |
| [2] | Sharma, A. K.; Sharma, R.; Saxena, M. Spectroscopic and Biocidal activities of environmentally safe Agrochemicals. J Biochem. Tech., 2018, 7(3), 1139-1147. |
| [3] | Kapoor, P.; Fahmi, N.; Singh, R.V. Microwave assisted synthesis, spectroscopic, electrochemical and DNA cleavage studies of lanthanide(III) complexes with coumarin based imines. Spectrochim. Acta A Mol. Biomol. Spectrosc., 2011, 83(1), 74-81. [http://dx.doi.org/10.1016/j.saa.2011.07.054] [PMID: 21903455] |
| [4] | Mathur, N.; Dobhal, M.P.; Jain, N. A comparative antibacterial analysis of substituted benzothiazole and substituted phenylthiourea complexes with copper metal. Int. J. Green Herb. Chem., 2017, 7(1), 1-6. |
| [5] | Raj, B.K.; Kumar, A. Synthesis, characterization and biocidal activity of some Schiff base and its metal complexes of Co (II), Cu (II) and Ni (II). Orient. J. Chem., 2013, 29(3), 1187-1191. [http://dx.doi.org/10.13005/ojc/290349] |
| [6] | Tank, P.; Sharma, A.K.; Sharma, R. Thermal behaviour and kinetics of copper (II) soaps and complexes derived from mustard and soyabeanOil. J. Anal. Pharm. Res., 2017, 4(2), 1-5. [http://dx.doi.org/10.15406/japlr.2017.04.00102] |
| [7] | Mathur, N.; Bargotya, S. Evaluation of novel N/S containing heterocyclic metal complexes as biologically potent agents. Res. J. Chem. Sci., 2018, 8(4), 30-34. |
| [8] | Bargotya, S.; Mathur, N.; Sharma, A.K. Antimicrobial & DNA cleavage studies of heterocyclic metal complexes“ISBN978-613-8-34627-2., 2018, |
| [9] | Sharma, S.; Sharma, R.; Sharma, A.K. Synthesis, characterization, and thermal degradation of Cu (II) surfactants for sustainable green chem. Asian J. Green Chem., 2017, 2(2), 129-140. [http://dx.doi.org/10.22631/ajgc.2017.95559.1015] |
| [10] | Sharma, A.K.; Sharma, S.; Sharma, R. Thermal degradation of Cu (II) metallic Soaps and their Characterizations. A Pharmaceutical Application Chron. Pharm. Sci., 2017, 1(5), 312-319. |
| [11] | Mishra, A.P.; Mishra, R.K.; Shrivastava, S.P. Structural and antimicrobial studies of coordination compounds of VO(II), Co(II), Ni(II) and Cu(II) with some Schiff bases involving 2-amino-4-chlorophenol. J. Serb. Chem. Soc., 2009, 74, 523-535. [http://dx.doi.org/10.2298/JSC0905523M] |
| [12] | Pillai, V.V.; Sreekanth, B. DNA binding and antimicrobial studies of Ag (II) and Cu (II) metalcomplexes containing mixed ligands of 1, 10- phenanthroline and 8-hydroxyuinoline. Int. J. Pharma. Bio. Sci., 2013, 4, 739-747. |
| [13] | Jain, P.; Kachhwaha, S.; Kothari, S.L. Chloroplast ultra structure, photosynthesis and enzyme activities in regenerated plants of Stevia rebaudiana (Bert.) Bertoni as influenced by copper sulphate in the medium. Indian J. Exp. Biol., 2014, 52(9), 898-904. [PMID: 25241590] |
| [14] | Mathur, N.; Bargotya, S.; Manna, B.; Kasana, A. Antifungal screening and oxidation behaviour of some microwave assisted benzothaizine drugs. Int. J. Chem. Sci., 2014, 12(2), 533-546. |
| [15] | Sharma, A.K.; Sharma, R.; Saxena, M. Synthesis, spectroscopic and fungicidal studies of Cu (II) soaps derived from groundnut and sesame oils and their urea complexes. Bulletin Pure Appl. Sci., 2017, 36(2), 26-37. [http://dx.doi.org/10.5958/2320-320X.2017.00004.8] |
| [16] | Mathur, N.; Bargotya, S. Advances in the Research of Novel N/S Containing Metal Complexes as Potent Antioxidants. J. Appl. Chem., 2017, 10(8), 18-23. |
| [17] | Sharma, A. K.; Sharma, R.; Saxena, M. Biomedical and antifungal application of Cu(II) soaps and its urea complexes derived from various oils. Open Access J. Trans. Med. Res., 2018, 2(2), 40-43. |
| [18] | Bargotya, S.; Mathur, N.; Sharma, A.K. Physico-chemical analysis of environmentally safe complexes of copper “ISBN978-613-8-34672-2”., 2018, |
| [19] | Mathur, N.; Bargotya, S. DNA–Binding and cleavage studies of macrocyclic metal complexes containing heteroatomic ligands. Chem. Sci. Trans., 2016, 5(1), 117-124. |
| [20] | Bhutra, R.; Sharma, R.; Sharma, A.K. Synthesis, Characterization and fungicidal activities of Cu (II) surfactants derived from groundnut and mustard oils treated at high temperatures. J. Inst. Chemists (India), 2018, 90(3), 66-80. |
| [21] | Mathur, N.; Sharma, A.K. Synthesis of Hetero-cyclic compounds for Bio-medical Applications., 2018, |
| [22] | Joram, A.; Sharma, R.; Sharma, A.K. Thermal degradation of complexes derived from Cu (II) groundnut soap (Arachis hypogaea) and Cu (II) sesame soap (Sesamum indicum). Z. Phys. Chem., 2018, 232(4), 459-470. [http://dx.doi.org/10.1515/zpch-2017-1073] |
| [23] | Sharma, A.K.; Sharma, R.; Gangwal, A. Antifungal activities and characterization of some new environmentally safe Cu (II) surfactants substituted 2-amino-6-methyl benzothiazole. Open Phar. Sci. J., 2018, 5, 3-12. |
| [24] | Khan, S.; Sharma, R.; Sharma, A.K. Antifungal Activities of Copper Surfactants derived from Neem (AzadirectaIndica) and Karanj (Pongamiapinnata) Oils: A Pharmaceutical Application. Glob. J. Pharmaceu. Sci., 2017, 3(4), 1-6. |
| [25] | Mathur, N.; Heda, L.C.; Mathur, V.K.; Saxena, P. Study of CLSI-M44-A Disk Diffusion method for determining the susceptibility of candida species against novel complexes derived from copper stearate with 2-amino benzothiazoles. Tenside Surf. Det., 2011, 48(1), 1-5. [http://dx.doi.org/10.3139/113.110099] |
| [26] | Sharma, R.; Sharma, A.K. Natural edible oils: Comparative health aspects of sesame, coconut, mustard (Rape Seed) and Groundnut (Peanut) A Biomedical Approach. Biomed. J. Sci. Tech. Res., 2017, 1(5), 1-4. |
| [27] | Mathur, N.; bargotya, S.; Mathur, R. Thermogravimetric analysis of microwave assisted novel macromolecular complexes of metal surfactants. J. App. Chem., 2014, 3(2), 712-719. |
| [28] | Tank, P.; Sharma, R.; Sharma, A.K. A Pharmaceutical approach & Antifungal activities of Copper Soaps with their N & S donor complexes derived from Mustard and Soyabean oils. Glob. J. Pharmaceu Sci., 2017, 3(4), 1-6. |
| [29] | Mathur, N.; Manna, B. TGA analysis of transition metal complexes derived from phenothiazine ligands. Int. J. Sci. Eng. Res., 2018, 6(3), 47-57. |
| [30] | Sharma, A.K.; Sharma, R.; Gangawal, A. Biomedical and fungicidal application of copper surfactants derived from pure fatty acid. Org. Med. Chem. I J., 2018, 5(5), 1-3 . |
| [31] | Mathur, N.; Bargotya, S. A facile synthesis and biological evaluation of some macro cyclic copper complexes. Int. J. Pharm. Sci. Res., 2015, 6(6), 2538-2345. |
| [32] | Mathur, N.; Bargotya, S. Synthesis characterization and plant growth regulatory activity of metal complexes with some bioactive ligands. World J. Pharm. Pharm. Sci., 2016, 5(11), 945-955. |
| [33] | Sharma, A.K.; Saxena, M.; Sharma, R. Fungicidal activities and characterization of novel biodegradable Cu (II) surfactants derived from lauric acid. Open Chem. J., 2018, 5, 89-101. [http://dx.doi.org/10.2174/1874842201805010089] |
| [34] | Mathur, N.; Jain, N.; Sharma, A.K. Biocidal activities of substituted benzothiazole of copper surfactants over Candida albicans & Trichoderma harzianumon Muller Hinton Agar. Open Pharm. Sci. J., 2018, 5, 24-35. [http://dx.doi.org/10.2174/1874844901805010024] |
| [35] | Bhutra, R.; Sharma, R.; Sharma, A.K. Antimicrobial studies and characterization of copper surfactants derived from various oils treated at high temperatures by P.D.A. technique. Open Phar Sci. J., 2018, 5, 36-40. [http://dx.doi.org/10.2174/1874844901805010036] |