- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Fungicidal Activities and Characterization of Novel Biodegradable Cu (II) Surfactants Derived from Lauric Acid
Arun Kumar Sharma1, *, Meenakshi Saxena2, Rashmi Sharma3
Abstract
Introduction:
Colloidal systems are extremely widespread in nature and are of great practical importance in our daily life. Surfactants are very important in modern engineering and pharmaceutical soap and the complexes of soaps with different ligands are used in almost all sectors of national economy due to the formation of micelles in solutions and high surface activity i.e. the ability of their molecules to form surface adsorption layers. For this purpose, first time we thought about the synthesis of copper surfactants/soaps and their complexation by N/S donor ligands.
Methods and Materials:
In this paper, we report the synthesis of copper laurate thiourea by conventional methods and its characterization by elemental analysis, IR, NMR, ESR spectral studies. In order to understand their biological aspects and application of these surfactants/complexes as antifungal agents, astudy has also been conducted in the field of biochemistry. In order to understand their biological aspects with special reference to fungicidal activities, three different fungi namely Aspergillus alternaria, Aspergillus Fumigatus and Aspergillus niger were taken and tested by different concentrations of copper laurate soap and its thiourea complex by P.D.A. (Potato dextrose agar) technique.
Conclusion:
Biological studies of these compounds will also provide an important account of information about their industrial utilization.
Article Information
Identifiers and Pagination:
Year: 2018Volume: 5
First Page: 89
Last Page: 101
Publisher Id: CHEM-5-89
DOI: 10.2174/1874842201805010089
Article History:
Received Date: 5/5/2018Revision Received Date: 29/7/2018
Acceptance Date: 7/8/2018
Electronic publication date: 26/10/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Govt. P.G. College, Jhalawar-326001, Rajasthan, India; Tel: +919352669899; E-mail: sharmaarun423@gmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 5-5-2018 |
Original Manuscript | Fungicidal Activities and Characterization of Novel Biodegradable Cu (II) Surfactants Derived from Lauric Acid | |
1. INTRODUCTION
The chemistry of macrocyclic N and S donor ligands and their complexes with transition metal ions has been an interesting and fascinating area of research activity all over the world since the last few decades. The continued interest to proliferate structural novelties of such complexes is due to their wide application in medicinal, biochemical, bioinorganic, environment, industrial and photochemistry [1Tank, P.; Sharma, A.K.; Sharma, R. Thermal behaviour and kinetics of copper (II) soaps and complexes derived from mustard and soyabean Oil. J. Anal. Pharm. Res., 2017, 4(2), 1-5.
[http://dx.doi.org/10.15406/japlr.2017.04.00102] ]. Surface-active agents or surfactants are of great practical importance in our daily life, on account of their interesting behavior at surfaces and interfaces. They not only accumulate at the surface but also change the properties of surfaces [2Sharma, S.; Sharma, R.; Sharma, A.K. Synthesis, characterization, and thermal degradation of Cu (II) surfactants for sustainable green chem. Asian J. Green Chem., 2017, 2(2), 129-140.
[http://dx.doi.org/10.22631/ajgc.2017.95559.1015] ]. Synthesis of Cu (II) soaps and their complexes derived from various edible and non-edible oils will provide fundamental information regarding their colloidal-chemical behavior and fungicidal activities [3Bhati, S.K.; Kumar, A. Synthesis of new substituted azetidinoyl and thiazolidinoyl-1,3,4-thiadiazino (6,5-b) indoles as promising anti-inflammatory agents. Eur. J. Med. Chem., 2008, 43(11), 2323-2330.
[http://dx.doi.org/10.1016/j.ejmech.2007.10.012] [PMID: 18063224] ]. Cu (II) soaps have gained importance due to their use in wood preservation, pesticidal and fungicidal activities, foaming, wetting, detergency, emulsification, plants lubrication etc [4Joram, A.; Sharma, R.; Sharma, A.K Thermal degradation of complexes derived from Cu (II) groundnut soap (Arachishypogaea) and Cu (II) sesame soap (Sesamumindicum), Z phys. Chem., 2008, 232(4), 459-470.
[http://dx.doi.org/10.1515/zpch-2017-1073] ]. Physical properties on Cu (II) soaps and their complexes such as ultrasonic studies [5Khan, S.; Sharma, R.; Sharma, A.K. Acoustic studies and other acoustic parameters of Cu(II) soap derived from non-edible Neem oil (Azadirectaindica), in Non-aqueous media at 298. 15 Acta Ac united Ac104, 2018, 277-283.
[http://dx.doi.org/10.3813/AAA.919170] , 6Sharma, A.K.; Saxena, M.; Sharma, R. Ultrasonic studies of Cu (II) soaps derived from groundnut and sesame oils, Tenside. Surf. Det., 2018, 55(2), 127-134.
[http://dx.doi.org/10.3139/113.110544] ], density, molar volume, apparent molar volume [7Tank, P.; Sharma, R.; Sharma, A.K. Micellar features and various interactions of copper soap complexes derived from edible mustard oil in benzene at 303.15 K. Curr. Phy. Chem., 2018, 8(1), 46-57.
[http://dx.doi.org/10.2174/1877946808666180102152443] , 8Bhutra, R.; Sharma, R.; Sharma, A.K. Volumetric studies of copper soap derived from treated and untreated oils in benzene at 298. 15 K Bulletin of Pure and Applied Sciences Section-C-Chemistry, 2018, 37(2), 33-44.
[http://dx.doi.org/10.5958/2320-320X.2018.00028.6] ], viscometric studies [9Sharma, A.K.; Khan, S.; Sharma, R. Viscometric behaviour and micellar studies of Cu (II) surfactant derived from Neem (AzadirectaIndica)) oil in methanol-benzene mixture at 298. 15 K. Global J. Eng. Sci. Res., 2018, 9-16.
[http://dx.doi.org/10.5281/zenodo.1288395] ], TGA analysis [10Sharma, A.K.; Sharma, S.; Sharma, R. Thermal degradation of Cu (II) metallic Soaps and their Characterizations. Pharmaceuti. Appl. Chronicles Pharmaceuti. Sci, 2017, 1(5), 312-319.], photochemical degradation [11Sharma, S.; Sharma, R.; Heda, L.C.; Sharma, A.K. Kinetic parameters and photo degradation studies of copper soap derived from soybean Oil using ZnO as a photo catalyst in solid and solution phase. J. Inst. Chemists (India), 2017, 89(4), 119-136.] and antifungal studies [12Rashmi, S; Arun, K S Natural Edible Oils: Comparative health Aspects of sesame, coconut, mustard (rape seed) and Groundnut (peanut). Biomed. Approach. Biomed J. Sci. & TechRes, 2017, 1(5) BJSTR.MS.ID.000441 https://doi.org/10.26717/BJSTR.2017.01.000441] were earlier reported to provide a fundamental and informative account of micellar features of Cu (II) soaps derived from various oils i.e. soyabean, mustard, sesame, groundnut, neem and karanj in pure benzene, varying concentration of benzene-methanol solvent system. The ligands used during the present investigation thiourea have been very well reported to possess biocidal activity [13Tank, P.; Sharma, R.; Sharma, A. K. A Pharmaceutical approach & Antifungal activities of copper soaps with their N & S donor complexes derived from mustard and soyabean oils. Glob. J. Pharmac. Sci, 2017, 3(4) GJPPS.MS.ID.555619 https://doi.org/10.19080/GJPPS.2017.03.555619]. For this purpose, the Cu (II) laurate soap and its thiourea complex have been synthesized by conventional methods and characterized by the various physicochemical and spectral analyses. Our continuing interest in the search for better fungicides and bactericides has led us to synthesize some new complexes derived from copper-laurate soaps with the above mention ligand and screen them for their fungicidal activities.
2. EXPERIMENTAL
Copper laurate soap was prepared by direct metathesis of the corresponding potassium soap standard reported methods [14Saxena, M.; Sharma, R.; Sharma, A. K. Micellar Features of Cu (II) Surfactants derived from Edible Oils. LAP Lambert Academic Publishing Germany, 2017.]. Copper soap-thiourea complex was prepared by taking copper soap and thiourea in a molar ratio (1:1). 0.005 moles of ligand (thiourea) was dissolved in 2-3 ml of ethyl alcohol and 0.005 moles of copper (II) soap derived from lauric acid was dissolved in 10-15 ml of benzene and solution of thiourea was added to it. The above reaction mixture was then heated for 1.5 h. Separated solid complex was filtered, washed with hot water and alcohol and dried in vacuum over fused calcium chloride. The dried sample was purified and re-purified with hot benzene. In general, the solid complex (90% yield) with bluish green periphery was obtained (Table 1). The compound is soluble in ethanol, methanol, benzene and other organic solvents and insoluble in water. The complex is quite stable at room temperature up to 170°C. On the basis of elemental analysis, the complex has been assigned with the composition and Cu2[CnH2n+1COO]4L2 suggested 1:1 type stoichiometry. Elemental analysis, was performed at RSIC, CDRI Lucknow, U.P. India. The newly synthesized agrochemicals are abbreviated as follows:
- Copper laurate soap (CLrtS)
- Copper laurate thiourea complex (CLrtT)
The proposed structure of soap and complex have shown in Figs. (1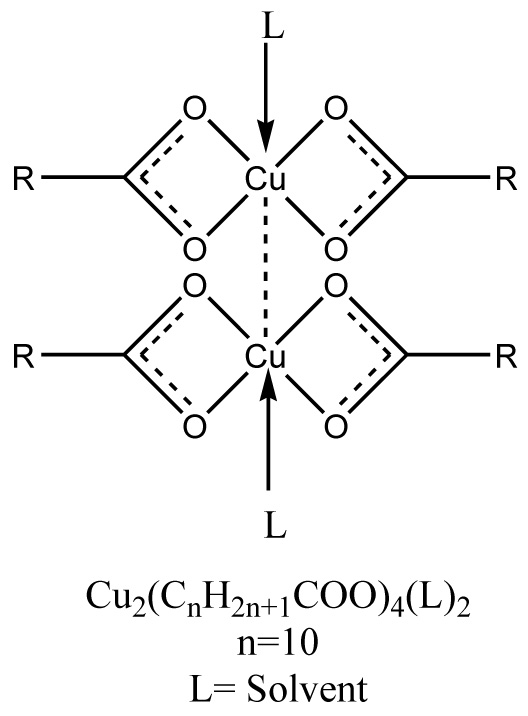 , 2
, 2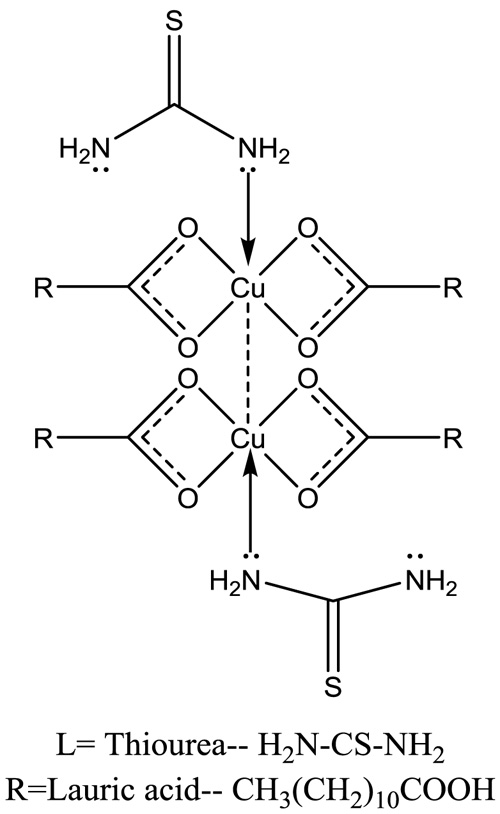 ).
).
 |
Fig. (1) Proposed structure of CLrtS (Cu2(CnH2n+1COO)4(L2), n= 11, L= solvent. |
 |
Fig. (2) Proposed structure of CLrtT. |
2.1. Materials and Instrumentation
A Perkin-Elmer spectrum-2000 Fourier transform IR spectrophotometer (USA) was used to obtain the IR spectra between 400 and 4000 cm−1 CDRI Lucknow U.P. India. The samples were prepared in pellet form using spectroscopic grade KBr. 1H-NMR spectra were recorded by multinuclear FTNMR spectrometer Model Advance-II (Bruker). The instrument was equipped with a cryo-magnet of field strength 9.4 T. Its 1H frequency was 400 MHz at CDRI Lucknow U.P. India. The ESR spectra of the complexes were recorded at X-Band at a modulation frequency of 100KHZ at liquid nitrogen temperature. TCNE was used as the field marker at RSIC, IIT. Powai, Mumbai, India. The g values for ESR signal are calculated by the formula-(Eq 1).
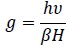 |
(1) |
Where υ = Frequencies of Band in KHz
B = Bohr magneton
H = Magnetic field, h = Planks constant.
On the basis of gll and g ┴, the gav and G values were calculated using the following (Eq 2 and 3).
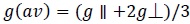 |
(2) |
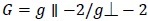 |
(3) |
The general laboratory techniques followed in the course of this investigation are as suggested by Boothand Hawks worth [15Booth, C. “Methods in Microbiology” AcadPress.N.Y. Vol-4, 795P, (1971).] as follows:
2.2. Sterilization of Glasswares
For a biological activity, the glassware were thoroughly washed and cleaned with chromic acid, followed by washing with distilled water. Now they were sterilized by keeping them in a hot air oven at 160 0C for 24h. All operations concerning inoculation are done in a completely sterilized chamber.
2.3. Inoculation
The artificial induction of micro-organism into a medium is called inoculation. The latter is the most fundamental technique for studying the growth characteristics of micro-organisms and for the transfer and maintenance of culture under aseptic condition.
2.4. Preparation of Slant
Agar slants were prepared to inoculate microbial culture. To prepare agar slant, the required number of culture tubes were taken and about 12 to 15 ml of liquefied agar medium was poured in each of them. The tubes were now cotton-plugged and sterilized in an autoclave. After the sterilization was over, the tubes were taken out and were placed in slanting (stopping) position for some time, the tubes got cooled and the medium in them was solidified resulting in a sloppy surface.
2.5. Culture Media Used
In preparing a Culture medium for any micro-organism, the primary goal is to provide a balanced mixture of the nutrient that will permit good growth. Additionally, the culturing of micro-organisms requires Careful Control of various environmental factors which normally are maintained within narrow culture media.
2.6. Preparation of PDA
The culture medium used for the growth of the organism in the present study was natural media Potato Dextrose Agar (Abbreviated as ‘PDA”).For the preparation of culture media standard reported procedure [16Kumar, A.; Rajput, C.S. Synthesis and anti-inflammatory activity of newer quinazolin-4-one derivatives. Eur. J. Med. Chem., 2009, 44(1), 83-90.
[http://dx.doi.org/10.1016/j.ejmech.2008.03.018] [PMID: 18501478] ].
2.7. Test Organism
The test organisms used in the present study were Alternaria alternate, Aspergillus fumigatus and Aspergillus niger which were isolated from their natural habitat (plants, debris) and then purified, characterized and identified.
2.8. Fungicidal Testing
The antifungal activity of the copper soaps and their complexes derived from saturated fatty acid under study was checked by the agar plate technique as reported in the literature [17Sharma, A.K.; Sharma, R.; Gangwal, A.K. Antifungal activities and characterization of some new environmentally safe Cu (II) surfactants substituted 2-amino-6-methyl benzothiazole. Open Phar Sci. J. , 2018, 1-11 3-12 https://DOI:10.2174/187484490180501]. The growth of fungus was measured by recording the total area of the fungal colony
The data were statistically analyzed according to the following formula [18Mathur, N.; Bargotya, S. DNA binding and cleavage activities of macro cyclic metal complexes containing heteroatomic ligand Chem. Sci. Trans., 2016, 5(1), 117-124.].
Fungal Growth Formula = 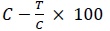 (2)
(2)
(% Inhibition)
C = Total area of the fungal colony in control plats after 2 days.
T = Total area of the fungal colony in the test tube after 2 days.
3. RESULTS AND DISCUSSION
3.1. IR Spectra
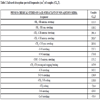
The absorption bands observed at 2926 cm-1 and 2859 cm-1 are assigned to the antisymmetric and symmetric stretching for –CH2 group (methylene) of the soap segment present in the complex. The absorption bands observed at 2961 cm-1 are due to –CH3 antisymmetric stretching of lauric acid. A strong absorption band at 1551 cm-1 is due to carboxylate ion COO-, C-O antisymmetric respectively. The peaks corresponding to –CH3 and –CH2 have been seen at 1118 cm-1 and 726 cm-1respectively. Cu- O stretching bands have been shown in the region of 476.4 cm-1 similar observation was reported by Khan et al., [19Khan, S.; Sharma, R.; Sharma, A.K. Antifungal activities of copper surfactants derived from Neem (AzadirectaIndica) and Karanj (Pongamiapinnata) Oils: A pharmaceutical application. Glob. J. Pharmaceu. Sci., 2017, 3(4) GJPPS.MS.ID.555616]. The absorption bands observed at 3333 cm-1 and 3180 cm-1 are due to the N-H antisymmetric stretching and N-H symmetric stretching of NH2 group. Other strong peaks in the region of 1479 cm-1 are due to the CH2 bending group. The absorption band 1714 cm-1 was found to be representative of amide group of >C=O. The C-N stretching band of primary amide was observed at 1413 cm-1. Apart from these absorption corresponding to the ligand moiety, a peak in the region of 1266 cm-1 N=C=S stretching of ligand thiourea in the complex was observed. Due to the C=S stretching group in an absorption in the region of 624 cm-1(Table 2). On the basis of the above observations, it can be safely assumed that complexation of copper laurate soap has been done with thiourea ligand (Fig. 3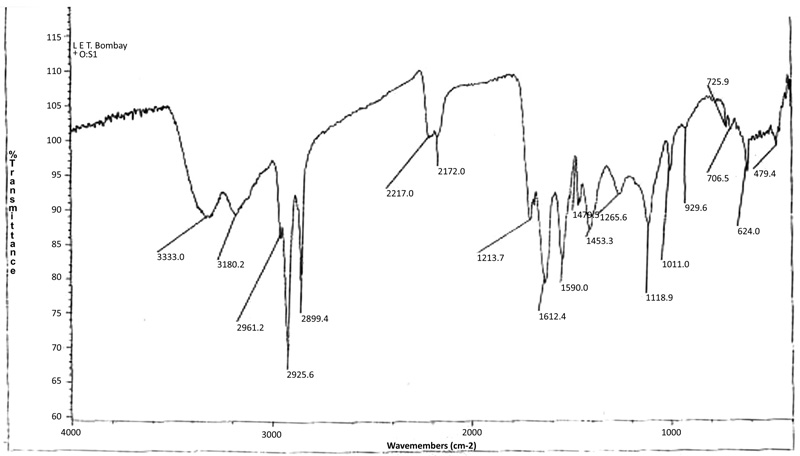 ).
).
 |
Fig. (3) IR Spectra of complex (CLrtT). |
3.2. NMR Spectra
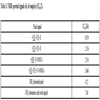
NMR spectra of Cu (II) laurate thiourea complex show a signal of aliphatic –CH3 proton attached -CH2-R group at nearly δ-0.979 and – CH2 proton attached to -CH2-R group which shows a signal at δ-1.256. Other signal observed is co-responding to the –CH2 proton attached to –CH2(COO)2Cu group and is observed at δ-2.354. All these peaks are due to the saturated fatty acid content of the soap in the complex. A broad peak is observed in the spectra of the complex at δ-2.354 due to the presence of –NH2 protons (Table 3). This peak indicated co-ordination through the -NH2 group of thiourea segment to the metal atom of the soap segment. A very weak signal is observed at δ-7.84 in the spectra, which may be due to tautomerism [20Mahajan, k.; Swami, M.; Singh, R.V. Microwave synthesis, spectral studies, antimicrobial approach, and coordination behavior of antimony(III) and bismuth(III) compounds with benzothiazoline. Russ. J. Coord. Chem., 2009, 35, 179-180.
[http://dx.doi.org/10.1134/S1070328409030038] ] present in the complex (Fig. 4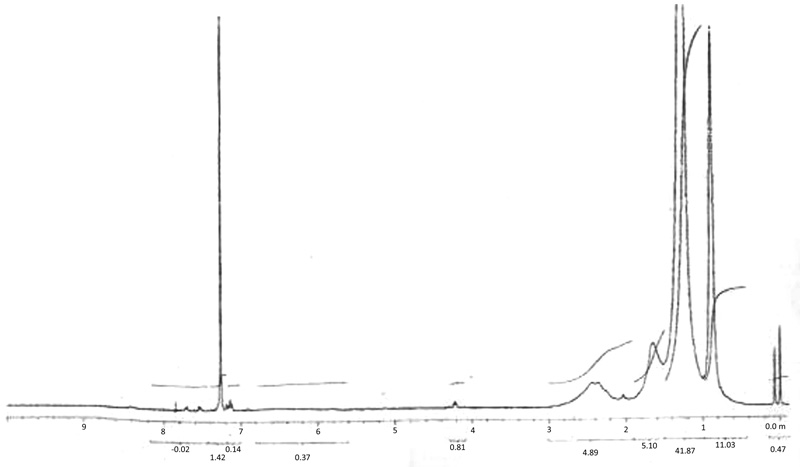 ).
).
 |
Fig. (4) NMR Spectra of complex (CLrtT). |
3.3. ESR Spectra
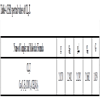
The value of ESR parameters for the complex is given in the Table 4. A perusal of Table 4 shows that the values of g1 g11, g are greater than the value of g i.e. 2.0027. This indicates that the distortion from the regular octahedron has taken place in the shape of the complex. Also the trend g11>g for complex indicates that the unpaired electron is most likely in the dx2-y2 orbit of Cu (II) giving the ground state. This fact supports that complex possesses elongated octahedral geometry. It is well known that g11 is a moderately sensitive function for indicating co-valency [21Garg, B.S.; Kumar, D.N.; Singh, R.V. Spectral studies of complexes of nickel (II) with tetradentate schiff bases having N2O2 donor groups. Spectrochim. Acta., 2003, 59A, 229-234.
[http://dx.doi.org/10.1016/S1386-1425(02)00142-7] ]. Thus the values of g11 are of the covalent character of the metal-ligand bond. Earlier magnetic studies about Cu (II) soap confirmed the binuclear configuration in solid state [22Sharma, A.K.; Saxena, M.; Sharma, R. Synthesis, spectroscopic and fungicidal studies of Cu (II) soaps derived from groundnut and sesame oils and their urea complexes Bulletin of Pure and Applied Sciences 2017, 36(2), 26-37.
[http://dx.doi.org/10.5958/2320-320X.2017.00004.8] ] (Fig. 5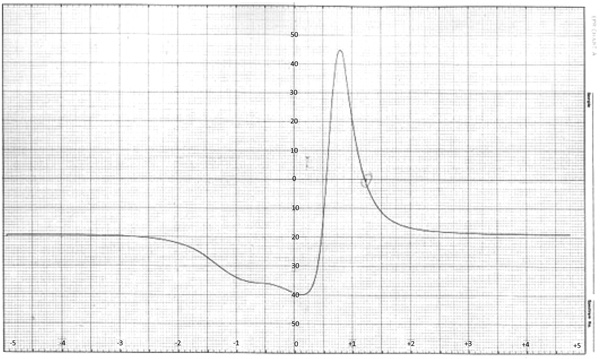 ).
).
 |
Fig. (5) ESR Spectra of complex (CLrtT). |
3.4. Fungicidal Activities
A perusal of Figs. (6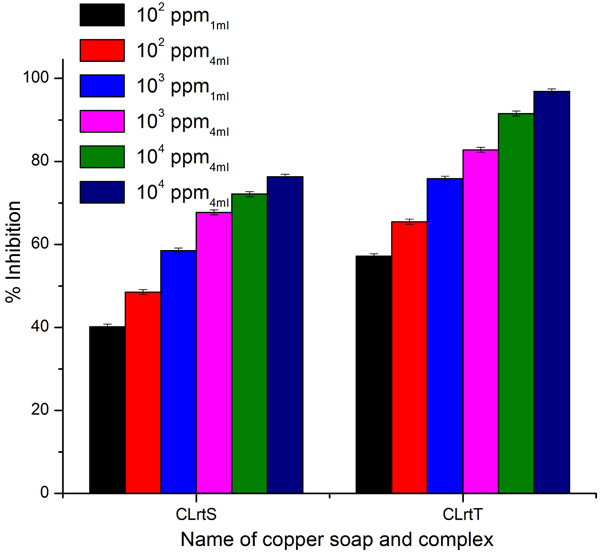 -8
-8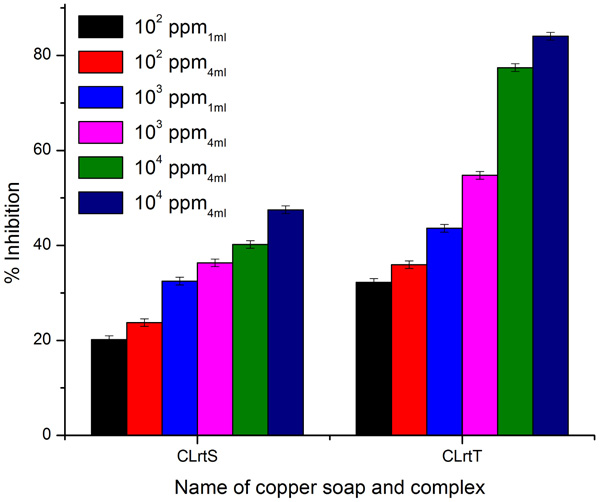 ) reveals that the complex shows higher activity than pure soap suggesting that complex is more powerful antifungal agent. Thiourea and other N,S,O etc. containing compounds are able to enhance the performance of copper soap. These results show that the Cu (II) soap complex of ligands is much toxic than the copper-soap themselves. The enhanced activity of synthesis of the complex as compared to copper soap can possibly be explained on the basis of chelate formation in the presence of donor atoms, basicity as well as the structural compatibility with molecular nature of the toxic moiety [23Neha Mathur and SonlataBargotya. A facile synthesis and biological evalution of some macrocyclic copper complexes. IJPSR, 2015, 6(6), 2538-2545., 24 Sharma, A, K. Saxena, M. Sharma R.: Synthesis, Spectroscopic and Biocidal activities of environmentally safe Agrochemicals, J. Biochem. tech., 2018, 7(3), 1139-1147.]. The final conclusion suggests that the appearance of enhanced activity may be due to a synergistic mechanism i.e. the soap is less active but on complexation shows more activity in combination with thiourea. The studies suggest that the Cu (II) ions in soaps may be responsible for the enhancement of the activity against fungi. The evaluation of anti-fungal studies further revealed that fungitoxicity of the complex also depends on the nature of metal ions [25 Sujamol, M.S., Athira, C.J., Sindhu, Y., Mohanan, K.:Synthesis, spectroscopic characterization, electrochemical behaviour and thermal decomposition studies of some transition metal complexes with an azo derivative,Spectrochim. Acta. 75A(2010) 106-112; https://DOI:10.1016/j.saa.2009.09.050]. The chelating reduces the polarity of central metal ion mainly because of the partial attaining of its positive charge with the donor groups and possible ring. Such chelating increases the lipophilic character of the central atom, which subsequently favors its permeation through the lipid layer of the cell membrane [26Bhutra, R.; Sharma, R.; Sharma, A.K. Synthesis, Characterization and fungicidal activities of Cu (II) surfactants derived from groundnut and mustard oils treated at high temperatures. J. Inst. Chemists (India), 2018, 90(3), 66-80.]. Their efficiency increases with their concentration. Thus, it is evident that concentration plays a vital role in increasing the degree of inhibition So, fungicidal screening data revealed that at a lower concentration of the inhibition of growth is less as compared to higher concentration [27 Mishra, A.P., Mishra, R.K., Shrivastava, S.P.:Structural and antimicrobial studies of coordination compounds of VO(II), Co(II), Ni(II) and Cu(II) with some Schiff bases involving 2-amino-4-chlorophenol, J. Serb. Chem. Soc., 74(2009) 523-535; https://DOI: 10.2298/JSC0905523M-29Mathur, N.; Ahmad, I.; Kasana, A. SonlataBargotya, Biplab Manna, Biological Activities of Some New Environmentally Safe 2 Aminobenzothiazole Complexes of Copper (II). Derived Under Microwave Irradiation, IAASCA, 2013, 5(1), 37-42.]. On the basis of fungicidal screening data, it was revealed that the complex shows lower activities for Alternaria alternate and Aspergillus fumigatus in comparison with Aspergillus niger (Figs. 9a
) reveals that the complex shows higher activity than pure soap suggesting that complex is more powerful antifungal agent. Thiourea and other N,S,O etc. containing compounds are able to enhance the performance of copper soap. These results show that the Cu (II) soap complex of ligands is much toxic than the copper-soap themselves. The enhanced activity of synthesis of the complex as compared to copper soap can possibly be explained on the basis of chelate formation in the presence of donor atoms, basicity as well as the structural compatibility with molecular nature of the toxic moiety [23Neha Mathur and SonlataBargotya. A facile synthesis and biological evalution of some macrocyclic copper complexes. IJPSR, 2015, 6(6), 2538-2545., 24 Sharma, A, K. Saxena, M. Sharma R.: Synthesis, Spectroscopic and Biocidal activities of environmentally safe Agrochemicals, J. Biochem. tech., 2018, 7(3), 1139-1147.]. The final conclusion suggests that the appearance of enhanced activity may be due to a synergistic mechanism i.e. the soap is less active but on complexation shows more activity in combination with thiourea. The studies suggest that the Cu (II) ions in soaps may be responsible for the enhancement of the activity against fungi. The evaluation of anti-fungal studies further revealed that fungitoxicity of the complex also depends on the nature of metal ions [25 Sujamol, M.S., Athira, C.J., Sindhu, Y., Mohanan, K.:Synthesis, spectroscopic characterization, electrochemical behaviour and thermal decomposition studies of some transition metal complexes with an azo derivative,Spectrochim. Acta. 75A(2010) 106-112; https://DOI:10.1016/j.saa.2009.09.050]. The chelating reduces the polarity of central metal ion mainly because of the partial attaining of its positive charge with the donor groups and possible ring. Such chelating increases the lipophilic character of the central atom, which subsequently favors its permeation through the lipid layer of the cell membrane [26Bhutra, R.; Sharma, R.; Sharma, A.K. Synthesis, Characterization and fungicidal activities of Cu (II) surfactants derived from groundnut and mustard oils treated at high temperatures. J. Inst. Chemists (India), 2018, 90(3), 66-80.]. Their efficiency increases with their concentration. Thus, it is evident that concentration plays a vital role in increasing the degree of inhibition So, fungicidal screening data revealed that at a lower concentration of the inhibition of growth is less as compared to higher concentration [27 Mishra, A.P., Mishra, R.K., Shrivastava, S.P.:Structural and antimicrobial studies of coordination compounds of VO(II), Co(II), Ni(II) and Cu(II) with some Schiff bases involving 2-amino-4-chlorophenol, J. Serb. Chem. Soc., 74(2009) 523-535; https://DOI: 10.2298/JSC0905523M-29Mathur, N.; Ahmad, I.; Kasana, A. SonlataBargotya, Biplab Manna, Biological Activities of Some New Environmentally Safe 2 Aminobenzothiazole Complexes of Copper (II). Derived Under Microwave Irradiation, IAASCA, 2013, 5(1), 37-42.]. On the basis of fungicidal screening data, it was revealed that the complex shows lower activities for Alternaria alternate and Aspergillus fumigatus in comparison with Aspergillus niger (Figs. 9a -c
-c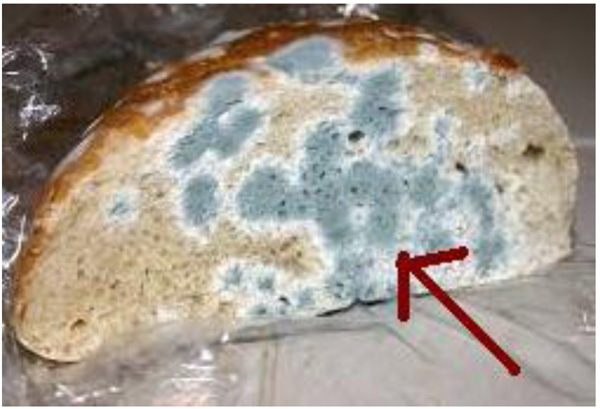 ). For Aspergillus niger, the complex’s efficiency increases with its concentration. Thus it is evident that the concentration plays a vital role in the degree of inhibition. The study suggests that copper laurate soap is the least fungi toxic (% inhibition lowest) whereas its thiourea complex which shows the highest inhibition. The activity of copper soap and complex derived from lauric acid is found to increase in the order.
). For Aspergillus niger, the complex’s efficiency increases with its concentration. Thus it is evident that the concentration plays a vital role in the degree of inhibition. The study suggests that copper laurate soap is the least fungi toxic (% inhibition lowest) whereas its thiourea complex which shows the highest inhibition. The activity of copper soap and complex derived from lauric acid is found to increase in the order.
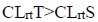 |
It has also been observed that, in general, copper complex derived from lauric acid shows higher % inhibition for Aspergillus niger as compared to Aspergillus fumigatus and Alternaria alternata.
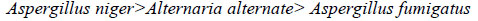 |
Higher concentration shows higher % inhibition when compared to the lower concentration.
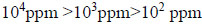 |
 |
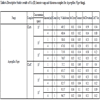
The results of ANOVA for the antifungal activities for all sops complexes are shown in Table 5. The predicted R2 is in reasonable agreement and closer to 1.0. This confirms that the experimental data are well satisfactory. The descriptive statistics results of CLrtS and ClrtT showed in Tables 6-8 confirm satisfactory results in triplet for all the fungi studied earlier in our laboratory and other studies conducted by scientists [30Sharma, A.K.; Sharma, R.; Saxena, M. 2018. Biomedical and antifungal application of Cu(II) soaps and its urea complexes derived from various oils. Open access J Trans Med res 2(2) 40-43. https://Doi:10.15406/oajtmr.2018.02.00033]. The findings are similar to our studies on different systems [31Sharma, A.K.; Sharma, R.; Gangwal, A. Biomedical and fungicidal application of copper surfactants derived from pure fatty acid, Organic & Medicinal Chem., IJ 5(5): OMCIJ.MS.ID.555680 (2018) 1-4 https://DOI:10.19080/OMCIJ.2018.05.555680.]. The result is statistically significant, by the standards of the study, due to p < F.
 |
Fig. (6) Antifungal activity of ClrtS and CLrtT for fungi Aspergillus Niger. |
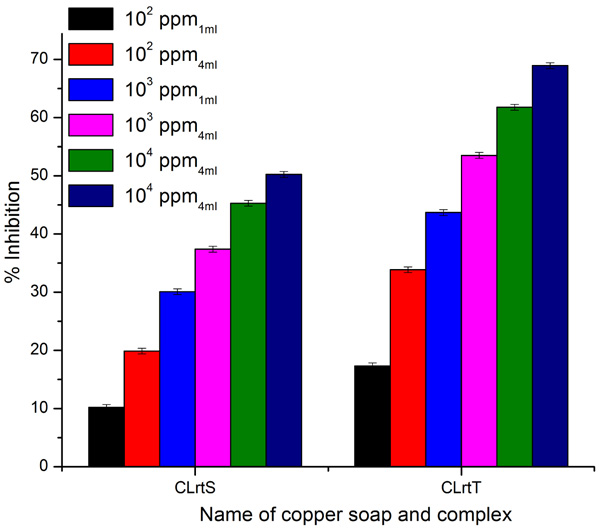 |
Fig. (7) Antifungal activity of ClrtS and CLrtT for fungi Aspergillus fumigatus. |
 |
Fig. (8) Antifungal activity of ClrtS and CLrtT for fungi Alternaria Alternata. |
 |
Fig. (9a) Presence of Aspergillus fumigatus on papaya fruit. |
 |
Fig. (9b) Presence of Alternaria Alternata on tomato. |
 |
Fig. (9c) Presence of Aspergillus Niger on bread. |
CONCLUSION
Biologically potent compounds are one of the most important classes of materials for the upcoming generations. This initiates a task for current chemistry to synthesize compounds that show promising activity as therapeutic agents with lower toxicity. Therefore, a substantial research is needed for their discovery and improvement. Transition metal complexes share an important place in this regards. Further, it is evidenced that complexation of the above metal ions with nitrogen and sulfur donor ligands increases the efficiency of biocidal activity. The antifungal activities of copper soap and its complex have been evaluated by the P.D.A. method. A scrutiny of the results reveals that the transition metal complexes showed antifungal activity better than soap, suggesting that the complexes are more powerful agents. Thiourea and other N and S containing compounds are able to enhance the performance of copper soaps.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors pay their sincere gratitude to UGC for the financial assistance and Principal, S.D. Govt. college Beawar and S.P.C. Govt. College Ajmer, Rajasthan (India) for providing necessary research facilities to accomplish this study. IIT, Mumbai is gratefully acknowledged for providing with the spectral data.
REFERENCES
| [1] | Tank, P.; Sharma, A.K.; Sharma, R. Thermal behaviour and kinetics of copper (II) soaps and complexes derived from mustard and soyabean Oil. J. Anal. Pharm. Res., 2017, 4(2), 1-5. [http://dx.doi.org/10.15406/japlr.2017.04.00102] |
| [2] | Sharma, S.; Sharma, R.; Sharma, A.K. Synthesis, characterization, and thermal degradation of Cu (II) surfactants for sustainable green chem. Asian J. Green Chem., 2017, 2(2), 129-140. [http://dx.doi.org/10.22631/ajgc.2017.95559.1015] |
| [3] | Bhati, S.K.; Kumar, A. Synthesis of new substituted azetidinoyl and thiazolidinoyl-1,3,4-thiadiazino (6,5-b) indoles as promising anti-inflammatory agents. Eur. J. Med. Chem., 2008, 43(11), 2323-2330. [http://dx.doi.org/10.1016/j.ejmech.2007.10.012] [PMID: 18063224] |
| [4] | Joram, A.; Sharma, R.; Sharma, A.K Thermal degradation of complexes derived from Cu (II) groundnut soap (Arachishypogaea) and Cu (II) sesame soap (Sesamumindicum), Z phys. Chem., 2008, 232(4), 459-470. [http://dx.doi.org/10.1515/zpch-2017-1073] |
| [5] | Khan, S.; Sharma, R.; Sharma, A.K. Acoustic studies and other acoustic parameters of Cu(II) soap derived from non-edible Neem oil (Azadirectaindica), in Non-aqueous media at 298. 15 Acta Ac united Ac104, 2018, 277-283. [http://dx.doi.org/10.3813/AAA.919170] |
| [6] | Sharma, A.K.; Saxena, M.; Sharma, R. Ultrasonic studies of Cu (II) soaps derived from groundnut and sesame oils, Tenside. Surf. Det., 2018, 55(2), 127-134. [http://dx.doi.org/10.3139/113.110544] |
| [7] | Tank, P.; Sharma, R.; Sharma, A.K. Micellar features and various interactions of copper soap complexes derived from edible mustard oil in benzene at 303.15 K. Curr. Phy. Chem., 2018, 8(1), 46-57. [http://dx.doi.org/10.2174/1877946808666180102152443] |
| [8] | Bhutra, R.; Sharma, R.; Sharma, A.K. Volumetric studies of copper soap derived from treated and untreated oils in benzene at 298. 15 K Bulletin of Pure and Applied Sciences Section-C-Chemistry, 2018, 37(2), 33-44. [http://dx.doi.org/10.5958/2320-320X.2018.00028.6] |
| [9] | Sharma, A.K.; Khan, S.; Sharma, R. Viscometric behaviour and micellar studies of Cu (II) surfactant derived from Neem (AzadirectaIndica)) oil in methanol-benzene mixture at 298. 15 K. Global J. Eng. Sci. Res., 2018, 9-16. [http://dx.doi.org/10.5281/zenodo.1288395] |
| [10] | Sharma, A.K.; Sharma, S.; Sharma, R. Thermal degradation of Cu (II) metallic Soaps and their Characterizations. Pharmaceuti. Appl. Chronicles Pharmaceuti. Sci, 2017, 1(5), 312-319. |
| [11] | Sharma, S.; Sharma, R.; Heda, L.C.; Sharma, A.K. Kinetic parameters and photo degradation studies of copper soap derived from soybean Oil using ZnO as a photo catalyst in solid and solution phase. J. Inst. Chemists (India), 2017, 89(4), 119-136. |
| [12] | Rashmi, S; Arun, K S Natural Edible Oils: Comparative health Aspects of sesame, coconut, mustard (rape seed) and Groundnut (peanut). Biomed. Approach. Biomed J. Sci. & TechRes, 2017, 1(5) BJSTR.MS.ID.000441 https://doi.org/10.26717/BJSTR.2017.01.000441 |
| [13] | Tank, P.; Sharma, R.; Sharma, A. K. A Pharmaceutical approach & Antifungal activities of copper soaps with their N & S donor complexes derived from mustard and soyabean oils. Glob. J. Pharmac. Sci, 2017, 3(4) GJPPS.MS.ID.555619 https://doi.org/10.19080/GJPPS.2017.03.555619 |
| [14] | Saxena, M.; Sharma, R.; Sharma, A. K. Micellar Features of Cu (II) Surfactants derived from Edible Oils. LAP Lambert Academic Publishing Germany, 2017. |
| [15] | Booth, C. “Methods in Microbiology” AcadPress.N.Y. Vol-4, 795P, (1971). |
| [16] | Kumar, A.; Rajput, C.S. Synthesis and anti-inflammatory activity of newer quinazolin-4-one derivatives. Eur. J. Med. Chem., 2009, 44(1), 83-90. [http://dx.doi.org/10.1016/j.ejmech.2008.03.018] [PMID: 18501478] |
| [17] | Sharma, A.K.; Sharma, R.; Gangwal, A.K. Antifungal activities and characterization of some new environmentally safe Cu (II) surfactants substituted 2-amino-6-methyl benzothiazole. Open Phar Sci. J. , 2018, 1-11 3-12 https://DOI:10.2174/187484490180501 |
| [18] | Mathur, N.; Bargotya, S. DNA binding and cleavage activities of macro cyclic metal complexes containing heteroatomic ligand Chem. Sci. Trans., 2016, 5(1), 117-124. |
| [19] | Khan, S.; Sharma, R.; Sharma, A.K. Antifungal activities of copper surfactants derived from Neem (AzadirectaIndica) and Karanj (Pongamiapinnata) Oils: A pharmaceutical application. Glob. J. Pharmaceu. Sci., 2017, 3(4) GJPPS.MS.ID.555616 |
| [20] | Mahajan, k.; Swami, M.; Singh, R.V. Microwave synthesis, spectral studies, antimicrobial approach, and coordination behavior of antimony(III) and bismuth(III) compounds with benzothiazoline. Russ. J. Coord. Chem., 2009, 35, 179-180. [http://dx.doi.org/10.1134/S1070328409030038] |
| [21] | Garg, B.S.; Kumar, D.N.; Singh, R.V. Spectral studies of complexes of nickel (II) with tetradentate schiff bases having N2O2 donor groups. Spectrochim. Acta., 2003, 59A, 229-234. [http://dx.doi.org/10.1016/S1386-1425(02)00142-7] |
| [22] | Sharma, A.K.; Saxena, M.; Sharma, R. Synthesis, spectroscopic and fungicidal studies of Cu (II) soaps derived from groundnut and sesame oils and their urea complexes Bulletin of Pure and Applied Sciences 2017, 36(2), 26-37. [http://dx.doi.org/10.5958/2320-320X.2017.00004.8] |
| [23] | Neha Mathur and SonlataBargotya. A facile synthesis and biological evalution of some macrocyclic copper complexes. IJPSR, 2015, 6(6), 2538-2545. |
| [24] | Sharma, A, K. Saxena, M. Sharma R.: Synthesis, Spectroscopic and Biocidal activities of environmentally safe Agrochemicals, J. Biochem. tech., 2018, 7(3), 1139-1147. |
| [25] | Sujamol, M.S., Athira, C.J., Sindhu, Y., Mohanan, K.:Synthesis, spectroscopic characterization, electrochemical behaviour and thermal decomposition studies of some transition metal complexes with an azo derivative,Spectrochim. Acta. 75A(2010) 106-112; https://DOI:10.1016/j.saa.2009.09.050 |
| [26] | Bhutra, R.; Sharma, R.; Sharma, A.K. Synthesis, Characterization and fungicidal activities of Cu (II) surfactants derived from groundnut and mustard oils treated at high temperatures. J. Inst. Chemists (India), 2018, 90(3), 66-80. |
| [27] | Mishra, A.P., Mishra, R.K., Shrivastava, S.P.:Structural and antimicrobial studies of coordination compounds of VO(II), Co(II), Ni(II) and Cu(II) with some Schiff bases involving 2-amino-4-chlorophenol, J. Serb. Chem. Soc., 74(2009) 523-535; https://DOI: 10.2298/JSC0905523M |
| [28] | Bhutra, R.; Sharma, R.; Sharma, A.K. Fungicidal Activities of Cu (II) Soaps Derived From Various Oils Treated at High Temperature for Biomedical Use. SAJ Biotechnol, 2018, 5(103), 1-6. |
| [29] | Mathur, N.; Ahmad, I.; Kasana, A. SonlataBargotya, Biplab Manna, Biological Activities of Some New Environmentally Safe 2 Aminobenzothiazole Complexes of Copper (II). Derived Under Microwave Irradiation, IAASCA, 2013, 5(1), 37-42. |
| [30] | Sharma, A.K.; Sharma, R.; Saxena, M. 2018. Biomedical and antifungal application of Cu(II) soaps and its urea complexes derived from various oils. Open access J Trans Med res 2(2) 40-43. https://Doi:10.15406/oajtmr.2018.02.00033 |
| [31] | Sharma, A.K.; Sharma, R.; Gangwal, A. Biomedical and fungicidal application of copper surfactants derived from pure fatty acid, Organic & Medicinal Chem., IJ 5(5): OMCIJ.MS.ID.555680 (2018) 1-4 https://DOI:10.19080/OMCIJ.2018.05.555680. |