- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Magnetic Properties of One- and Two-Dimensional Functional Materials: Oxygen Molecules Encapsulated in Single-Walled Carbon Nanotubes and Copper Ions Embedded into Phthalocyanine Sheets
Masayuki Hagiwara1, *, Takanori Kida1, Kazuyuki Matsuda2, Haruka Kyakuno2, Yutaka Maniwa3, Zentaro Honda4, Yuya Sakaguchi4, Makoto Tashiro4, Masamichi Sakai4, Takeshi Fukuda4, Norihiko Kamata4, Kouichi Okunishi5
Abstract
Background:
In this paper, we report on the topics of one-dimensional (1D) and two-dimensional (2D) functional materials. Single-Walled Carbon Nanotubes (SWCNTs) are seamless hollow cylinders made of hexagonal lattice graphite sheets. The SWCNTs have attracted considerable attention due to the applicability of their enclosed nanospaces to engineering, and many types of guest materials are encapsulated inside their 1D space, expecting unusual properties. The poly Transition Metal (TM) phthalocyanine, in which phthalocyanine units are extended in two dimensions by sharing benzene rings, is one of the examples of the TM containing 2D carbon materials. Because of strong correlation between localized d-electrons in the TM atom and delocalized π-electrons on the poly phthalocyanine frame, it is expected that spin-polarized conduction, which is useful for the spintronic applications.
Objectives:
The objective of the first work is to synthesize SWCNTs encapsulating oxygen molecules having spin one, whose O-O bond directions are aligned to the longitudinal direction of the SWCNTs. The objective of the second work is to synthesize Poly Cu Phthalocyanine (PCuPc) through a bottom-up method by using copper octacyanophthalocyanine as a building block and to elucidate its crystal structure and magnetic properties.
Methods:
SWCNTs with inner diameter of ca 0.8 nm were prepared by the CoMoCAT method, and encapsulated together with oxygen molecules (~400 Torr) into a high-purity quartz tube. To subtract the background signals of the SWCNTs and the quartz tube, we prepared the same SWCNTs inducing He gas after evacuating oxygen molecules. Magnetization measurements of these SWCNTs samples were conducted by means of a SQUID magnetometer and a pulse magnet using an induction method. PCuPc were synthesized by a solid state reaction of octacyanophthalocyanine, tetracyanobenzene, and CuCl2·2H2O in glass ampoules sealed after evacuation. The as-synthesized samples were characterized using XRD analysis and TEM microscopy. Magnetization measurement of the samples were done by using a SQUID magnetometer.
Results:
The intrinsic magnetization data from oxygen molecules inside the SWCNTs (temperature and magnetic field dependence) show magnetic properties typical of the spin-one Heisenberg antiferromagnet named a Haldane magnet. PCuPc and its half-filling counterpart were obtained by solid state reaction. Both magnetic susceptibility and magnetization of PCuPc are larger than those of half-filling PCuPc, but the magnitudes of the former sample are about 1.5 times larger than those of the latter one, which is expected to be twice from the geometric superlattice structure.
Conclusion:
We have studied magnetic properties (magnetic susceptibility and magnetization) of oxygen molecules encapsulated into Single Walled Carbon Nanotubes (SWCNTs) with diameters of about 0.8 nm, regarded as a 1D functional magnetic material, and Poly Copper Phthalocyanine (PCuPc) and poly half-filling copper phthalocyanine (half-filling PCuPc), regarded as 2D functional magnetic materials.
Article Information
Identifiers and Pagination:
Year: 2019Volume: 6
Issue: Suppl-1, M4
First Page: 27
Last Page: 33
Publisher Id: CHEM-6-27
DOI: 10.2174/1874842201906010027
Article History:
Received Date: 13/01/2018Revision Received Date: 03/03/2018
Acceptance Date: 02/02/2019
Electronic publication date: 22/03/2019
Collection year: 2019
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Center for Advanced High Magnetic Field Science, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, Japan; Tel: +81-6-6850-6685; E-mails: hagiwara@ahmf.sci.osaka-u.ac.jp
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 13-01-2018 |
Original Manuscript | Magnetic Properties of One- and Two-Dimensional Functional Materials: Oxygen Molecules Encapsulated in Single-Walled Carbon Nanotubes and Copper Ions Embedded into Phthalocyanine Sheets | |
1. INTRODUCTION
The emergence of novel functionalities occasionally occurs by conventional materials or ions encapsulated in one-dimensional (1D) nanospaces and by those embedded in ideal two dimensional (2D) sheets because of restricted spaces and low dimensionality. In the following, we will describe how carbon-based materials, which are usually diamagnetic, have novel magnetic features by introducing magnetic atoms or ions into them.
1.1. Single-Walled Carbon Nanotubes (SWCNTs)
Single-Walled Carbon Nanotubes (SWCNTs) are formed by the sp2 bonding carbon atoms and are seamless hollow cylinders made of hexagonal lattice graphitic sheets [1Iijima, S. Helical microtubes of graphitic carbon. Nature, 1991, 354, 56-58.
[http://dx.doi.org/10.1038/354056a0] ]. The typical size ranges from less than one nanometer (nm) to a few nanometers in diameter and a few micrometers in length. As mentioned above, the SWCNTs have attracted much attention due to the applicability of their enclosed nanospaces and surfaces to engineering [2Smith, B.W.; Monthioux, M.; Luzzi, D.E. Encapsulated C60 in carbon nanotubes. Nature, 1998, 396, 323-324.
[http://dx.doi.org/10.1038/24521] -11Hanami, K.; Umesaki, T.; Matsuda, K.; Miyata, Y.; Kataura, H.; Okabe, Y.; Maniwa, Y. One-Dimensional Oxygen and Helical Oxygen Nanotubes inside Carbon Nanotubes. J. Phys. Soc. Jpn., 2010, 79, 023601-1-4.
[http://dx.doi.org/10.1143/JPSJ.79.023601] ]. Many types of guest materials are encapsulated inside their 1D space, and unusual phenomena have been observed. For example, water molecules inside SWCNTs named ice nanotubes [5Koga, K.; Gao, G.T.; Tanaka, H.; Zeng, X.C. Formation of ordered ice nanotubes inside carbon nanotubes. Nature, 2001, 412(6849), 802-805.
[http://dx.doi.org/10.1038/35090532] [PMID: 11518961] ] exhibit novel ferroelectric properties [8Mikami, F.; Matsuda, K.; Kataura, H.; Maniwa, Y. Dielectric properties of water inside single-walled carbon nanotubes. ACS Nano, 2009, 3(5), 1279-1287.
[http://dx.doi.org/10.1021/nn900221t] [PMID: 19385604] ].
The most interesting magnetic molecule is the oxygen molecule which is a unique magnetic homonuclear diatomic molecule having spin one (S=1) and has been studied for more than one hundred years [12Curie, M.P. Proprietes magnetiquesdes corps a diverse temperatures. Ann. Chim. Phys., 1895, 7(5), 289-405., 13Onnes, H.K.; Perrier, A. Magnetic researches. XII. The susceptibilityof solid oxygen in two forms. Leiden Comm., 1914, 139c, 25-32.]. Magnetic properties of oxygen molecules confined in nanospace have been studied considerably, e.g. in a graphitic slit-shaped nanospace [14Kanoh, H.; Kaneko, K. Magnetic Spin States of O2 Confined in a Graphite Slit-Shaped Nanospace at Low Temperature. J. Phys. Chem., 1996, 100, 755-759.
[http://dx.doi.org/10.1021/jp951986f] ] and in microporous metal-organic solids [15Mori, W.; Kobayashi, T.C.; Kurobe, J.; Amaya, K.; Narumi, Y.; Kumada, T.; Kindo, K.; Aruga-Katori, H.; Goto, T.; Miura, N.; Takamizawa, S.; Nakayama, H.; Yamaguchi, K. Magnetic properties of oxygen physisoebed in Cu-Trans-1,4-Cyclohexanedicarboxylic Acid Mol. Cryst. Liq. Cryst. (Phila. Pa.), 1997, 306, 1-7.
[http://dx.doi.org/10.1080/10587259708044542] -17Kobayashi, T.C.; Matsuo, A.; Suzuki, M.; Kindo, K.; Kitaura, R.; Matsuda, R.; Kitagawa, S. Magnetic properties of molecular oxygen adsorbed in micro-porous metal-organic solids. Prog. Theor. Phys. Suppl., 2005, 159, 271-279.
[http://dx.doi.org/10.1143/PTPS.159.271] ].
In previous numerical studies [11Hanami, K.; Umesaki, T.; Matsuda, K.; Miyata, Y.; Kataura, H.; Okabe, Y.; Maniwa, Y. One-Dimensional Oxygen and Helical Oxygen Nanotubes inside Carbon Nanotubes. J. Phys. Soc. Jpn., 2010, 79, 023601-1-4.
[http://dx.doi.org/10.1143/JPSJ.79.023601] ], five different structures are predicted depending on the diameter of the SWCNTs ranged from 0.6 to 2 nm. At the temperatures T < 10 K, the narrowest SWCNTs are filled with oxygen molecules having their O-O bond direction aligned to the longitudinal direction of the SWCNTs as shown in the inset of Fig. (1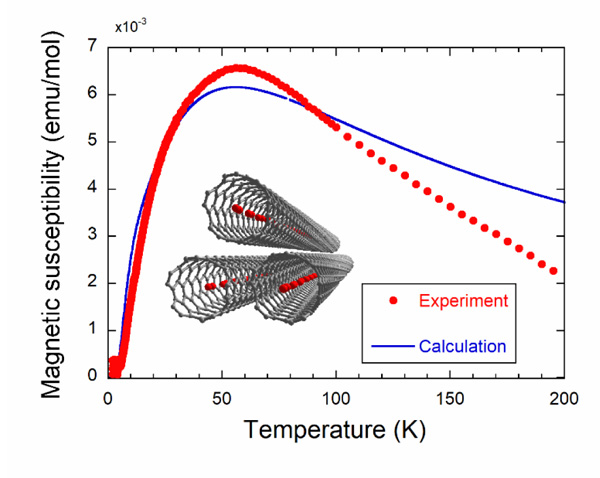 ). This geometry of oxygen molecule alignment is named 1DL and the exchange interaction between the neighboring oxygen molecules is expected to be antiferromagnetic [18van Hemert, M.C.; Wormer, P.E.S.; van der Avoird, A. Ab Initio calculation of the heisenberg exchange interaction between O2 molecules. Phys. Rev. Lett., 1983, 51, 1167-1170.
). This geometry of oxygen molecule alignment is named 1DL and the exchange interaction between the neighboring oxygen molecules is expected to be antiferromagnetic [18van Hemert, M.C.; Wormer, P.E.S.; van der Avoird, A. Ab Initio calculation of the heisenberg exchange interaction between O2 molecules. Phys. Rev. Lett., 1983, 51, 1167-1170.
[http://dx.doi.org/10.1103/PhysRevLett.51.1167] ].
1.2. Phthalocyanine Cu Poly Sheets (PCuPc)
Graphene is a 2D carbon material formed by the sp2 bonding carbon atoms [19Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science, 2004, 306(5696), 666-669.
[http://dx.doi.org/10.1126/science.1102896] [PMID: 15499015] ], and is intrinsically diamagnetic due to the pairing of σ and π electrons, except in the states at the zigzag edges [20Yoshizawa, K.; Okahara, K.; Sato, T.; Tanaka, K.; Yamabe, T. Molecular orbital study of pyrolytic carbons based on small cluster models. Carbon, 1994, 32, 1517-1522.
[http://dx.doi.org/10.1016/0008-6223(94)90147-3] , 21Enoki, T.; Takai, K. The edge state of nanographen and the magnetism of the edge-state spins. Solid State Commun., 2009, 149, 1144-1150.
[http://dx.doi.org/10.1016/j.ssc.2009.02.054] ]. Therefore, considerable efforts for further functionalization have been devoted to introduce Transition Metal (TM) atoms or ions with local magnetic moments into 2D carbon materials. Extensive studies have been made to explore TM-containing 2D carbon nanostructures, and one of them is Poly TM Phthalocyanine (PTMPc) [22Abel, M.; Clair, S.; Ourdjini, O.; Mossoyan, M.; Porte, L. Single layer of polymeric Fe-phthalocyanine: an organometallic sheet on metal and thin insulating film. J. Am. Chem. Soc., 2011, 133(5), 1203-1205.
[http://dx.doi.org/10.1021/ja108628r] [PMID: 21192107] -24Zhou, J.; Sun, Q. Magnetism of phthalocyanine-based organometallic single porous sheet. J. Am. Chem. Soc., 2011, 133(38), 15113-15119.
[http://dx.doi.org/10.1021/ja204990j] [PMID: 21838296] ]. The strong correlation between localized d electrons in TM atom or ion and delocalized π-electron on the poly phthalocyanine frame makes the spin-polarized conduction possible, which is useful for the spintronic application. Furthermore, the 2D porous structure of PTMPc ensures sufficient exposure of TM atoms to interact with reactants, and thus PTMPc may have functions applicable for the electro-catalysis and photo-catalysis. In addition, theoretical investigation suggests that the PTMPCs have high-temperature ferromagnetically or antiferromagnetically long ranged ordered state [24Zhou, J.; Sun, Q. Magnetism of phthalocyanine-based organometallic single porous sheet. J. Am. Chem. Soc., 2011, 133(38), 15113-15119.
[http://dx.doi.org/10.1021/ja204990j] [PMID: 21838296] ], which is applicable for magnetic functionalized-materials. In the case of TM=Cu2+ ions where the theory predicts antiferromagnetic coupling between Cu ions, we expect physical phenomena caused by quantum effects due to its small spin value (S=1/2) and two-dimensionality.
2. MATERIALS AND METHODS
2.1. Oxygen Molecules Encapsulated into SWCNTs
The SWCNTs sample with an inner diameter of ca 0.8 nm was prepared by the CoMoCAT method [25Kitiyanan, B.; Alvarez, W.E.; Harwell, J.H.; Resasco, D.E. Controlled production of single-wall carbon nanotubes by catalytic decomposition of CO on bimetallic Co-Mo catalysis. Chem. Phys. Lett., 2000, 317, 497-503.
[http://dx.doi.org/10.1016/S0009-2614(99)01379-2] ] and encapsulated together with oxygen molecules (~400 Torr) into a high-purity quartz tube. After conducting all the magnetic measurements of this sample, oxygen molecules were evacuated and replaced with helium gas. Then, we performed the same magnetic measurements as for the oxygen loaded sample. Since we used the same SWCNTs and quartz tube, the difference between the two samples is nearly identical to the contribution of oxygen molecules [26Hagiwara, M.; Ikeda, M.; Kida , T.; Mattsuda, K.; Tadera, S.; Kyakuno, H.; Yanagi, K.; Maniwa, Y.; Okunishi, K. Haldane state formed by oxygen molecules encapsulated in single-walled carbon nanotubes. J. Phys. Soc. Jpn., 2014, 83, 113706-1-4.
[http://dx.doi.org/10.7566/JPSJ.83.113706] ]. We assumed that the increase of observed magnetic susceptibility at low temperatures would arise from the oxygen molecules outside the SWCNT. Then, we subtracted this contribution, which is fitted by the Curie-Weiss law for S=1 with a Weiss temperature of -3 K, from the total magnetic susceptibility to get the intrinsic magnetic susceptibility of oxygen molecules inside the SWCNT. For the magnetization data, we subtracted the extrinsic magnetization calculated by assuming the S=1 Brillouin function from the total magnetization given by the difference between the magnetization curve with and without oxygen molecules. Powder X-ray diffraction experiments using synchrotron radiation X-rays were conducted at beamline BL8B of the Photon Factory in Japan in order to check the encapsulation of the oxygen molecules inside the SWCNTs, and we found the 1DL alignment of oxygen molecules depicted in the inset of Fig. (1 ).
).
2.2. Poly Cu Phthalocyanine
Poly Cu phthalocyanine and its half-filling counterpart were synthesized according to the methods described in Ref [27Honda, Z.; Sakaguchi, M.; Tashiro, Y.; Hagiwara, M.; Narumi, Y.; Kida, T.; Sakai, M.; Fukuda, T.; Kamata, N. Phthlocyanine based metal containing porous carbon sheet. Appl. Phys. Lett., 2017, 110, 133101-1-4.
[http://dx.doi.org/10.1063/1.4979030] ]. 1,2,4,5-tetracyanobenzen (tCB) and CuCl2·2H2O, which were purchased from Aldrich Chemical Company, Inc., were reacted at 240 °C for 4 hours in a minimal amount of sulfolane with the catalytic amounts of diaza-bicycloundecene [28Wöhrie, D.; Meyer, G. Polymere Phthalocyanine und ihre Vorstufen, 1 Reaktive oktafunktionelle Phthalocyanine aus 1,2,4,5-Tetracyanbenzol. Makromol. Chem., 1980, 181, 2127-2135.
[http://dx.doi.org/10.1002/macp.1980.021811010] ] to produce Cu Octacyano-Phthalocyanine (oCCuPc). Then, Poly Cu phthalocyanine (PCuPc) was synthesized by a solid-state reaction of oCCuPc, tCB, and CuCl2·2H2O in glass ampoules sealed after evacuation (1 Pa). The half-filling PCuPc was synthesized without CuCl2·2H2O. The sealed ampoules were placed into a conventional box furnace and maintained at 425 °C for 48 hours, and then allowed to cool naturally to room temperature. The crystal structures of the PCuPc products were analyzed by the powder XRD method with a Bruker D8 ADVANCE ECO diffractometer, and their TEM images were collected with an FEI Technai G2 20 microscope operated at 120 KeV [27Honda, Z.; Sakaguchi, M.; Tashiro, Y.; Hagiwara, M.; Narumi, Y.; Kida, T.; Sakai, M.; Fukuda, T.; Kamata, N. Phthlocyanine based metal containing porous carbon sheet. Appl. Phys. Lett., 2017, 110, 133101-1-4.
[http://dx.doi.org/10.1063/1.4979030] ]. From these analyses, the expected structures of PCuPc and half-filling PCuPc are depicted in Figs. (2a)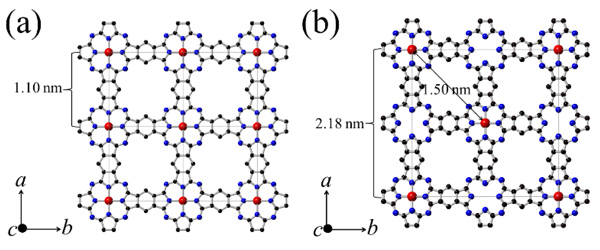 and (2b)
and (2b) , respectively.
, respectively.
3. EXPERIMENTAL
Magnetic susceptibility of oxygen molecules encapsulated into SWCNTs were measured with a Superconducting Quantum Interference Device (SQUID) magnetometer (Quantum Design MPMS-7XL). To obtain the magnetic susceptibility only from the oxygen molecules (oxygen susceptibility), we also measured the same SWCNTs filled with He gas into the quartz tube after evacuating the oxygen molecules. The intrinsic magnetic susceptibility of oxygen molecules confined into the SWCNTs was obtained by the aforementioned procedure. High field magnetization was measured with a pulsed magnet by an induction method. As mentioned in the subsection 2.1, we subtracted the paramagnetic contribution assuming a Brillouin function from the measured magnetization to get the intrinsic magnetization coming from the oxygen molecules inside the SWCNTs [26Hagiwara, M.; Ikeda, M.; Kida , T.; Mattsuda, K.; Tadera, S.; Kyakuno, H.; Yanagi, K.; Maniwa, Y.; Okunishi, K. Haldane state formed by oxygen molecules encapsulated in single-walled carbon nanotubes. J. Phys. Soc. Jpn., 2014, 83, 113706-1-4.
[http://dx.doi.org/10.7566/JPSJ.83.113706] ].
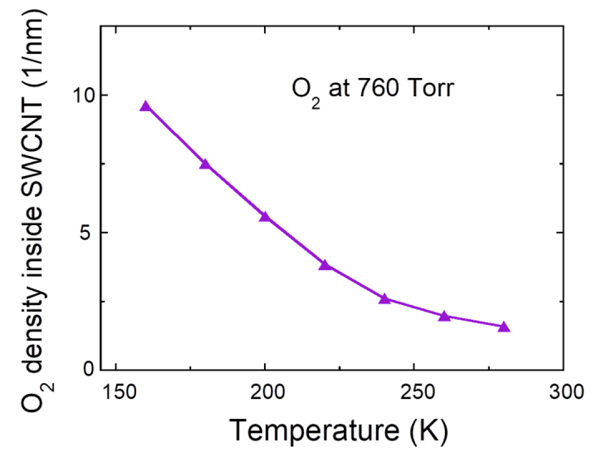 |
Fig. (3) Temperature dependence of oxygen density at 760 Torr inside the SWCNT with the diameter of 1.5 nm. |
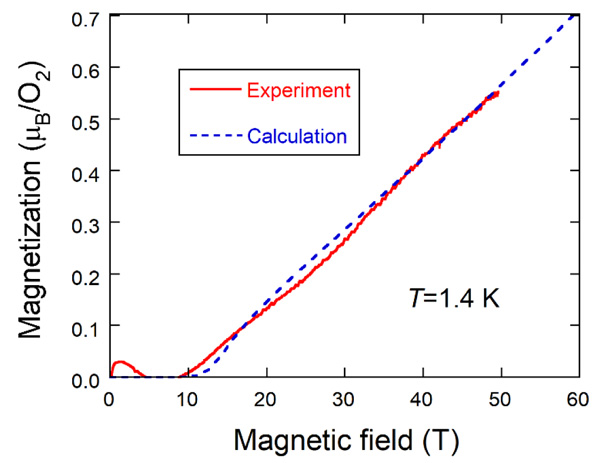 |
Fig. (4) Magnetization curve of oxygen molecules encapsulated into SWCNTs at 1.4 K. The broken line is the magnetization curve for the S=1 1D Heisenberg antiferromagnet. |
Magnetic susceptibilities and magnetization curves in magnetic fields of up to ±7 T of PCuPc and half-filling PCuPc were measured with the same SQUID magnetometer Diamagnetic corrections for these samples were not conducted.
4. RESULTS AND DISCUSSIONS
4.1. Oxygen Molecules Encapsulated into SWCNTs
The temperature dependence of intrinsic magnetic susceptibility (filled circles) is shown in Fig. (1 ). The intrinsic magnetic susceptibility is compared with the magnetic susceptibility calculated numerically for the S=1 1D Heisenberg antiferromagnet (solid line) [29Yamamoto, S. Quantum-classical crossover in temperature dependence of the magnetic susceptibility of haldane system. J. Phys. Soc. Jpn., 1995, 64, 4051-4052.
). The intrinsic magnetic susceptibility is compared with the magnetic susceptibility calculated numerically for the S=1 1D Heisenberg antiferromagnet (solid line) [29Yamamoto, S. Quantum-classical crossover in temperature dependence of the magnetic susceptibility of haldane system. J. Phys. Soc. Jpn., 1995, 64, 4051-4052.
[http://dx.doi.org/10.1143/JPSJ.64.4051] ]. The magnetic susceptibility shows a broad maximum at about 50 K, which is typical of the 1D antiferromagnet and falls down with decreasing temperature, reflecting a singlet ground state with an energy gap to the magnetic excited states. The difference in maximum values between the experimental and calculated magnetic susceptibilities may arise from the imprecise amount of oxygen molecules (6×10-6 mol) evaluated from the oxygen pressure (~400 Torr) and the inner volume of the quartz tube. In addition, the measured magnetic susceptibility decreases steeply at high temperatures and deviates largely from the calculated one. This must be caused by the escape of oxygen molecules absorbed inside the SWCNTs. Actually, oxygen density inside the SWCNTs with the diameter of 1.5 nm at 760 Torr decreases drastically upon heating as shown in Fig. (3 ). This observation solidifies the fact of the existence of oxygen molecules confined in the SWCNTs at low temperatures.
). This observation solidifies the fact of the existence of oxygen molecules confined in the SWCNTs at low temperatures.
Next, we show in Fig. (4 ) the intrinsic magnetization curve at 1.4 K (solid line) which is compared with the magnetization curve calculated for the S=1 1D Heisenberg antiferromagnet using the same parameter values as in the magnetic susceptibility fitting (broken line). Nearly zero- magnetization appears up to about 10 T and increases almost linearly, which is similar to the magnetization curve of Ni(C2H8N2)2NO2(ClO4), abbreviated as NENP [30Katsumata, K.; Hori, H.; Takeuchi, T.; Date, M.; Yamagishi, A.; Renard, J.P. Magnetization process of an S=1 linear-chain Heisenberg antiferromagnet. Phys. Rev. Lett., 1989, 63(1), 86-88.
) the intrinsic magnetization curve at 1.4 K (solid line) which is compared with the magnetization curve calculated for the S=1 1D Heisenberg antiferromagnet using the same parameter values as in the magnetic susceptibility fitting (broken line). Nearly zero- magnetization appears up to about 10 T and increases almost linearly, which is similar to the magnetization curve of Ni(C2H8N2)2NO2(ClO4), abbreviated as NENP [30Katsumata, K.; Hori, H.; Takeuchi, T.; Date, M.; Yamagishi, A.; Renard, J.P. Magnetization process of an S=1 linear-chain Heisenberg antiferromagnet. Phys. Rev. Lett., 1989, 63(1), 86-88.
[http://dx.doi.org/10.1103/PhysRevLett.63.86] [PMID: 10040439] ]. In contrast, oxygen molecules in micro-porous metal-organic solids exhibit a stepwise increase of magnetization [15Mori, W.; Kobayashi, T.C.; Kurobe, J.; Amaya, K.; Narumi, Y.; Kumada, T.; Kindo, K.; Aruga-Katori, H.; Goto, T.; Miura, N.; Takamizawa, S.; Nakayama, H.; Yamaguchi, K. Magnetic properties of oxygen physisoebed in Cu-Trans-1,4-Cyclohexanedicarboxylic Acid Mol. Cryst. Liq. Cryst. (Phila. Pa.), 1997, 306, 1-7.
[http://dx.doi.org/10.1080/10587259708044542] -17Kobayashi, T.C.; Matsuo, A.; Suzuki, M.; Kindo, K.; Kitaura, R.; Matsuda, R.; Kitagawa, S. Magnetic properties of molecular oxygen adsorbed in micro-porous metal-organic solids. Prog. Theor. Phys. Suppl., 2005, 159, 271-279.
[http://dx.doi.org/10.1143/PTPS.159.271] ]. A small hump observed below 4 T is caused by imperfect subtraction of extrinsic magnetization due to oxygen molecules outside the SWCNTs, which is made by assuming an S=1 Brillouin function. The agreement between experiment and calculation is considerably good. The zero-magnetization also indicates the existence of the energy gap between the singlet ground state and the excited magnetic state that is called the Haldane gap [31Haldane, F.D.M. Nonlinear field theory of large-spin heisenberg antiferromagnets: Semiclassically quantized solitons of the one-dimensional easy-axis néel state. Phys. Rev. Lett., 1983, 50, 1153-1156.
[http://dx.doi.org/10.1103/PhysRevLett.50.1153] ].
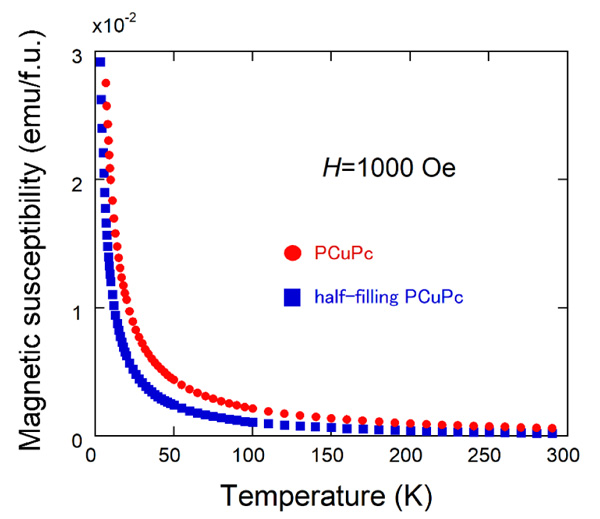 |
Fig. (5) Temperature dependence of magnetic susceptibilities of PCuPc and half-filling PCuPc. |
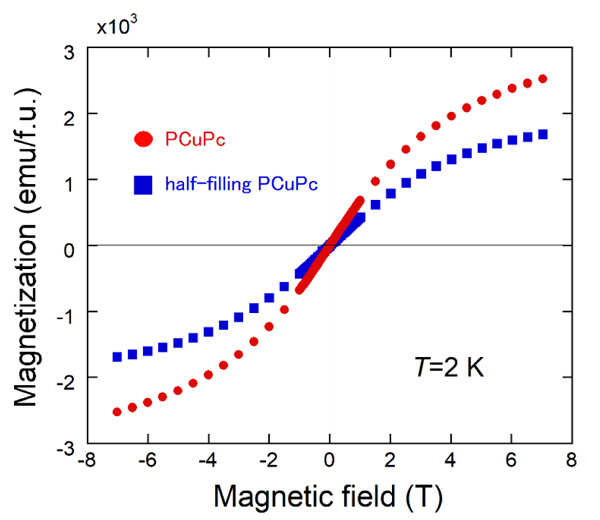 |
Fig. (6) Magnetization curves at 2K in magnetic fields of up to ±7 T for PCuPc and half-filling PCuPc samples. |
4.2. Poly Cu Phthalocyanine
Fig. (5 ) shows the temperature dependences of magnetic susceptibilities (M/H) of PCuPc (red filled circles) and half-filling PCuPc (blue filled squares) measured at 1 kOe. Both magnetic susceptibilities increase monotonically with decreasing temperature. Here, we used the following formula units: C20H4N8Cu, MW=419.9 g/mol for PCuPc, and C20H5N8Cu0.5, MW=389.1 g/mol for half-filling PCuPc. As expected from the crystal structures of these materials, magnetic susceptibility of the PCuPc is larger than that of the half-filling PCuPc. We expect the magnetic susceptibility of PCuPc twice larger than that of half-filling PCuPc. But the half filling geometric superlattice structure was observed partially in PCuPc by the TEM [23Koudia, M.; Abel, M. Step-by-step on-surface synthesis: from manganese phthalocyanines to their polymeric form. Chem. Commun. (Camb.), 2014, 50(62), 8565-8567.
) shows the temperature dependences of magnetic susceptibilities (M/H) of PCuPc (red filled circles) and half-filling PCuPc (blue filled squares) measured at 1 kOe. Both magnetic susceptibilities increase monotonically with decreasing temperature. Here, we used the following formula units: C20H4N8Cu, MW=419.9 g/mol for PCuPc, and C20H5N8Cu0.5, MW=389.1 g/mol for half-filling PCuPc. As expected from the crystal structures of these materials, magnetic susceptibility of the PCuPc is larger than that of the half-filling PCuPc. We expect the magnetic susceptibility of PCuPc twice larger than that of half-filling PCuPc. But the half filling geometric superlattice structure was observed partially in PCuPc by the TEM [23Koudia, M.; Abel, M. Step-by-step on-surface synthesis: from manganese phthalocyanines to their polymeric form. Chem. Commun. (Camb.), 2014, 50(62), 8565-8567.
[http://dx.doi.org/10.1039/C4CC02792B] [PMID: 24956396] ], and thus our PCuPc sample is a mixture of full-filling and half-filling PCuPcs, resulting in a smaller magnitude of magnetic susceptibility. We merely observed paramagnetic Curie-like behavior probably because of large distance between neighboring Cu ions, resulting in weak exchange interactions between them.
The magnetization curves at 2 K in magnetic fields of up to ±7 T of the PCuPc and the half-filling PCuPc samples are shown in Fig. (6 ). As in the magnetic susceptibilities, we use the unit of emu/f.u. for the magnetization. Both samples show gradual upward changes near ±7 T. As mentioned above, the PCuPc sample contains partly the half-filling area (Fig. 3b
). As in the magnetic susceptibilities, we use the unit of emu/f.u. for the magnetization. Both samples show gradual upward changes near ±7 T. As mentioned above, the PCuPc sample contains partly the half-filling area (Fig. 3b ) as in the half-filling PCuPc sample. Thus, the magnitude of the magnetization at 7 T of the PCuPc sample is about 1.5 times larger than that of the half-filling PCuPc sample. From the g-value (2.06) determined by multi-frequency electron spin resonance measurements, we expected the saturation value of magnetization (5750 emu/f.u.) for full-filling PCuPc. But the observed value is less than half of this expected value. As described by Honda et al., broadness of the XRD peak suggests the partial amorphous nature of the reaction product, and therefore Cu atoms may not be contained in some areas of our PCuPc sample [27Honda, Z.; Sakaguchi, M.; Tashiro, Y.; Hagiwara, M.; Narumi, Y.; Kida, T.; Sakai, M.; Fukuda, T.; Kamata, N. Phthlocyanine based metal containing porous carbon sheet. Appl. Phys. Lett., 2017, 110, 133101-1-4.
) as in the half-filling PCuPc sample. Thus, the magnitude of the magnetization at 7 T of the PCuPc sample is about 1.5 times larger than that of the half-filling PCuPc sample. From the g-value (2.06) determined by multi-frequency electron spin resonance measurements, we expected the saturation value of magnetization (5750 emu/f.u.) for full-filling PCuPc. But the observed value is less than half of this expected value. As described by Honda et al., broadness of the XRD peak suggests the partial amorphous nature of the reaction product, and therefore Cu atoms may not be contained in some areas of our PCuPc sample [27Honda, Z.; Sakaguchi, M.; Tashiro, Y.; Hagiwara, M.; Narumi, Y.; Kida, T.; Sakai, M.; Fukuda, T.; Kamata, N. Phthlocyanine based metal containing porous carbon sheet. Appl. Phys. Lett., 2017, 110, 133101-1-4.
[http://dx.doi.org/10.1063/1.4979030] ]. The same situation is observed in the half-filling PCuPc. We also observed paramagnetic magnetization curves in both samples due to the same reason as in the susceptibility measurements.
CONCLUSION
Novel magnetic properties would potentially emerge in conventional nonmagnetic materials when combining (encapsulating or embedding) with magnetic entities. As such trials, we have studied magnetic properties (magnetic susceptibility and magnetization) of oxygen molecules encapsulated into Single-Walled Carbon Nanotubes (SWCNTs) with diameters of about 0.8 nm, regarded as a one dimensional functional magnetic material, and Poly Copper Phthalocyanine (PCuPc) and poly half-filling copper phthalocyanine (half-filling PCuPc), regarded as two dimensional functional magnetic materials.
For the former, we observed magnetic susceptibility with a broad peak at about 50 K and a steep decrease towards zero upon further cooling below 50 K. The magnetization curve shows nearly zero magnetization up to 10 T and increases almost linearly. From these results, we have realized a Haldane magnet by arrayed oxygen molecules with spin one confined into the SWCNTs. This result paved the way to make noble magnetic materials using nano-spaced materials.
For the latter, we synthesized PCuPc and half-filling PCuPc using copper octa cyano phthalocyanine as a building block. Magnetic susceptibility and magnetization measurements were conducted for these samples. Both magnetic susceptibility and magnetization of PCuPc are larger than those of half-filling PCuPc, but the magnitudes of the former sample are about 1.5 times larger than those of the latter one, which is expected to be twice from the geometric superlattice structure. Furthermore, the saturation value of magnetization is also discussed, and the partial amorphous area suggested by the broad peak of XRD pattern may not contain Cu atoms in both samples.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
For the former topic, the XRD measurements were performed at BL8B of the Photon Factory in Japan. We thank T. Umezaki for his help with the XRD measurements. We also thank M. Ikeda, S. Tadera, and K. Yanagi for their help with the experiments. This work was supported by Grants-in-Aid for Scientific Research A (No. A25246006) and for Scientific Research on Priority Areas “New Materials Science Using Regulated Nano Spaces”, and by the Global COE Program named “Core Research and Engineering of Advanced Materials-Interdisciplinary Education Center for Materials Science (No. G10)” from the MEXT, Japan.
For the latter topic, this work was partially supported by Grants-in-Aid for Scientific Research (Grant Nos. 16K04926 and A25246006) from the MEXT, Japan. A part of this work was carried out at the Center for Advanced High Magnetic Field Science in Osaka University under the Visiting Researcher’s Program of the Institute for Solid State Physics, the University of Tokyo.
REFERENCES
| [1] | Iijima, S. Helical microtubes of graphitic carbon. Nature, 1991, 354, 56-58. [http://dx.doi.org/10.1038/354056a0] |
| [2] | Smith, B.W.; Monthioux, M.; Luzzi, D.E. Encapsulated C60 in carbon nanotubes. Nature, 1998, 396, 323-324. [http://dx.doi.org/10.1038/24521] |
| [3] | Calbi, M.M.; Cole, M.W.; Gatica, S.M.; Bojan, M.J.; Stan, G. Colloquium: Condensed phases of gases inside nanotube bundles. Rev. Mod. Phys., 2001, 73, 857-865. [http://dx.doi.org/10.1103/RevModPhys.73.857] |
| [4] | Satishkumar, B.C.; Taubert, A.; Luzzi, D.E. Filling single-wall carbon nanotubes with d- and f-metal chloride and metal nanowires. J. Nanosci. Nanotechnol., 2003, 3(1-2), 159-163. [http://dx.doi.org/10.1166/jnn.2003.181] [PMID: 12908245] |
| [5] | Koga, K.; Gao, G.T.; Tanaka, H.; Zeng, X.C. Formation of ordered ice nanotubes inside carbon nanotubes. Nature, 2001, 412(6849), 802-805. [http://dx.doi.org/10.1038/35090532] [PMID: 11518961] |
| [6] | Hummer, G.; Rasaiah, J.C.; Noworyta, J.P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature, 2001, 414(6860), 188-190. [http://dx.doi.org/10.1038/35102535] [PMID: 11700553] |
| [7] | Maniwa, Y.; Matsuda, K.; Kyakuno, H.; Ogasawara, S.; Hibi, T.; Kadowaki, H.; Suzuki, S.; Achiba, Y.; Kataura, H. Water-filled single-wall carbon nanotubes as molecular nanovalves. Nat. Mater., 2007, 6(2), 135-141. [http://dx.doi.org/10.1038/nmat1823] [PMID: 17237788] |
| [8] | Mikami, F.; Matsuda, K.; Kataura, H.; Maniwa, Y. Dielectric properties of water inside single-walled carbon nanotubes. ACS Nano, 2009, 3(5), 1279-1287. [http://dx.doi.org/10.1021/nn900221t] [PMID: 19385604] |
| [9] | Fan, X.; Dickey, E.C.; Eklund, P.C.; Williams, K.A.; Grigorian, L.; Buczko, R.; Pantelides, S.T.; Pennycook, S.J. Atomic arrangement of iodine atoms inside single-walled carbon nanotubes. Phys. Rev. Lett., 2000, 84(20), 4621-4624. [http://dx.doi.org/10.1103/PhysRevLett.84.4621] [PMID: 10990755] |
| [10] | Simon, F.; Kuzmany, H.; Náfrádi, B.; Fehér, T.; Forró, L.; Fülöp, F.; Jánossy, A.; Korecz, L.; Rockenbauer, A.; Hauke, F.; Hirsch, A. Magnetic Fullerenes inside Sigle-Wall Carbon Nanotubes. Phys. Rev. Lett., 2006, 97, 136801-1-4. [http://dx.doi.org/10.1103/PhysRevLett.97.136801] |
| [11] | Hanami, K.; Umesaki, T.; Matsuda, K.; Miyata, Y.; Kataura, H.; Okabe, Y.; Maniwa, Y. One-Dimensional Oxygen and Helical Oxygen Nanotubes inside Carbon Nanotubes. J. Phys. Soc. Jpn., 2010, 79, 023601-1-4. [http://dx.doi.org/10.1143/JPSJ.79.023601] |
| [12] | Curie, M.P. Proprietes magnetiquesdes corps a diverse temperatures. Ann. Chim. Phys., 1895, 7(5), 289-405. |
| [13] | Onnes, H.K.; Perrier, A. Magnetic researches. XII. The susceptibilityof solid oxygen in two forms. Leiden Comm., 1914, 139c, 25-32. |
| [14] | Kanoh, H.; Kaneko, K. Magnetic Spin States of O2 Confined in a Graphite Slit-Shaped Nanospace at Low Temperature. J. Phys. Chem., 1996, 100, 755-759. [http://dx.doi.org/10.1021/jp951986f] |
| [15] | Mori, W.; Kobayashi, T.C.; Kurobe, J.; Amaya, K.; Narumi, Y.; Kumada, T.; Kindo, K.; Aruga-Katori, H.; Goto, T.; Miura, N.; Takamizawa, S.; Nakayama, H.; Yamaguchi, K. Magnetic properties of oxygen physisoebed in Cu-Trans-1,4-Cyclohexanedicarboxylic Acid Mol. Cryst. Liq. Cryst. (Phila. Pa.), 1997, 306, 1-7. [http://dx.doi.org/10.1080/10587259708044542] |
| [16] | Kitaura, R.; Kitagawa, S.; Kubota, Y.; Kobayashi, T.C.; Kindo, K.; Mita, Y.; Matsuo, A.; Kobayashi, M.; Chang, H-C.; Ozawa, T.C.; Suzuki, M.; Sakata, M.; Takata, M. Formation of a one-dimensional array of oxygen in a microporous metal-organic solid. Science, 2002, 298(5602), 2358-2361. [http://dx.doi.org/10.1126/science.1078481] [PMID: 12493907] |
| [17] | Kobayashi, T.C.; Matsuo, A.; Suzuki, M.; Kindo, K.; Kitaura, R.; Matsuda, R.; Kitagawa, S. Magnetic properties of molecular oxygen adsorbed in micro-porous metal-organic solids. Prog. Theor. Phys. Suppl., 2005, 159, 271-279. [http://dx.doi.org/10.1143/PTPS.159.271] |
| [18] | van Hemert, M.C.; Wormer, P.E.S.; van der Avoird, A. Ab Initio calculation of the heisenberg exchange interaction between O2 molecules. Phys. Rev. Lett., 1983, 51, 1167-1170. [http://dx.doi.org/10.1103/PhysRevLett.51.1167] |
| [19] | Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science, 2004, 306(5696), 666-669. [http://dx.doi.org/10.1126/science.1102896] [PMID: 15499015] |
| [20] | Yoshizawa, K.; Okahara, K.; Sato, T.; Tanaka, K.; Yamabe, T. Molecular orbital study of pyrolytic carbons based on small cluster models. Carbon, 1994, 32, 1517-1522. [http://dx.doi.org/10.1016/0008-6223(94)90147-3] |
| [21] | Enoki, T.; Takai, K. The edge state of nanographen and the magnetism of the edge-state spins. Solid State Commun., 2009, 149, 1144-1150. [http://dx.doi.org/10.1016/j.ssc.2009.02.054] |
| [22] | Abel, M.; Clair, S.; Ourdjini, O.; Mossoyan, M.; Porte, L. Single layer of polymeric Fe-phthalocyanine: an organometallic sheet on metal and thin insulating film. J. Am. Chem. Soc., 2011, 133(5), 1203-1205. [http://dx.doi.org/10.1021/ja108628r] [PMID: 21192107] |
| [23] | Koudia, M.; Abel, M. Step-by-step on-surface synthesis: from manganese phthalocyanines to their polymeric form. Chem. Commun. (Camb.), 2014, 50(62), 8565-8567. [http://dx.doi.org/10.1039/C4CC02792B] [PMID: 24956396] |
| [24] | Zhou, J.; Sun, Q. Magnetism of phthalocyanine-based organometallic single porous sheet. J. Am. Chem. Soc., 2011, 133(38), 15113-15119. [http://dx.doi.org/10.1021/ja204990j] [PMID: 21838296] |
| [25] | Kitiyanan, B.; Alvarez, W.E.; Harwell, J.H.; Resasco, D.E. Controlled production of single-wall carbon nanotubes by catalytic decomposition of CO on bimetallic Co-Mo catalysis. Chem. Phys. Lett., 2000, 317, 497-503. [http://dx.doi.org/10.1016/S0009-2614(99)01379-2] |
| [26] | Hagiwara, M.; Ikeda, M.; Kida , T.; Mattsuda, K.; Tadera, S.; Kyakuno, H.; Yanagi, K.; Maniwa, Y.; Okunishi, K. Haldane state formed by oxygen molecules encapsulated in single-walled carbon nanotubes. J. Phys. Soc. Jpn., 2014, 83, 113706-1-4. [http://dx.doi.org/10.7566/JPSJ.83.113706] |
| [27] | Honda, Z.; Sakaguchi, M.; Tashiro, Y.; Hagiwara, M.; Narumi, Y.; Kida, T.; Sakai, M.; Fukuda, T.; Kamata, N. Phthlocyanine based metal containing porous carbon sheet. Appl. Phys. Lett., 2017, 110, 133101-1-4. [http://dx.doi.org/10.1063/1.4979030] |
| [28] | Wöhrie, D.; Meyer, G. Polymere Phthalocyanine und ihre Vorstufen, 1 Reaktive oktafunktionelle Phthalocyanine aus 1,2,4,5-Tetracyanbenzol. Makromol. Chem., 1980, 181, 2127-2135. [http://dx.doi.org/10.1002/macp.1980.021811010] |
| [29] | Yamamoto, S. Quantum-classical crossover in temperature dependence of the magnetic susceptibility of haldane system. J. Phys. Soc. Jpn., 1995, 64, 4051-4052. [http://dx.doi.org/10.1143/JPSJ.64.4051] |
| [30] | Katsumata, K.; Hori, H.; Takeuchi, T.; Date, M.; Yamagishi, A.; Renard, J.P. Magnetization process of an S=1 linear-chain Heisenberg antiferromagnet. Phys. Rev. Lett., 1989, 63(1), 86-88. [http://dx.doi.org/10.1103/PhysRevLett.63.86] [PMID: 10040439] |
| [31] | Haldane, F.D.M. Nonlinear field theory of large-spin heisenberg antiferromagnets: Semiclassically quantized solitons of the one-dimensional easy-axis néel state. Phys. Rev. Lett., 1983, 50, 1153-1156. [http://dx.doi.org/10.1103/PhysRevLett.50.1153] |





