- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Bioactive Compounds Journal
(Discontinued)
ISSN: 1874-8473 ― Volume 9, 2020
Evaluation of Antioxidant Potential of Stem and Leaf Extracts of Himalayan Tinospora cordifolia Hook.f. & Thomson
Geetanjali Upadhyay1, Lalit M. Tewari1, Geeta Tewari2, *, Neha Chopra1, Naveen C. Pandey1, Santosh K. Upadhyay3, Rekha Gahtori3
Abstract
Background:
Medicinal plants are considered a rich source of ingredients, which can be used in drug development and synthesis. Tinospora cordifolia (Wild.) Hook.f. & Thomson, commonly known as guduchi, heart-leaved moonseed and giloya is a herbaceous vine of the family Menispermaceae, has several beneficial properties including antioxidant activity.
Aim:
The present study was carried out to analyze the antioxidant activity of leaf and stem extracts of Tinospora cordifolia by using DPPH (1,1-Diphenyl-2-picrylhydrazyl) and ABTS (2,2´-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) free radical scavenging assays.
Materials and Methods:
Dried and powdered leaves and stem of T. cordifolia were extracted with methanol. Ascorbic acid was taken as standard. Total phenolic content was estimated by using Folin-ciocalteu's reagent while total flavonoid content by aluminium chloride reagent to find the correlation of polyphenols with antioxidant activity. ABTS assay of methanolic leaf and stem extracts showed the highest scavenging activity as compared to the DPPH assay.
Results:
Methanolic stem extract showed higher phenolic and flavonoid content along with antioxidant activity as compared to the methanolic leaf extract.
Conclusion:
The stem extract exhibited more antioxidant activity than the leaf extract with regards to the all parameters analyzed.
Article Information
Identifiers and Pagination:
Year: 2021Volume: 9
Issue: Suppl-1, M2
First Page: 2
Last Page: 8
Publisher Id: TOBCJ-9-2
DOI: 10.2174/1874847302109010002
Article History:
Received Date: 19/3/2020Revision Received Date: 19/4/2020
Acceptance Date: 11/5/2020
Electronic publication date: 18/06/2021
Collection year: 2021
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Chemistry, D. S. B. Campus, Kumaun University, Nainital, Uttarakhand, India; Tel: +09412438823; E-mail: geeta_k@rediffmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 19-3-2020 |
Original Manuscript | Evaluation of Antioxidant Potential of Stem and Leaf Extracts of Himalayan Tinospora cordifolia Hook.f. & Thomson | |
1. INTRODUCTION
Plants are one of the most important sources of medicines. They can synthesize different bioactive molecules such as phenols, flavonoids, vitamins, alkaloids, terpenoids, tannins, glycosides, quinones and many others. Most of the plants used for medicinal purposes have been identified and their uses are well documented and described by various authors [1Nadkarni KM. India material medica 1976.-3Saradamma I. All India co-ordinated research project on ethanobiology Final technique report RRI Drug Research, CCRAS, Government of India, Poojapura 1990.]. Medicinal plants have been used as natural medicines and they came into existence since prehistoric times. There are different ways in which plants have been found useful in medicines. The crude extract of plants has been used directly because of the presence of natural chemical constituents such as berberine, morphine, psilocin and vincristine [4Mittal J, Sharma MM, Batra A. Tinospora cordifolia a multipurpose medicinal plant- A review. J Med Plants Stud 2014; 2: 32-47.]. Medicinal plant research has increased all over the world and collected the immense potential of medicinal plants used in various traditional systems. The extraction and characterization of bioactive compounds from medicinal plants have resulted in the introduction of new drugs with high medicinal value [5Gangola S, Khati P. Parul, Bhatt P, Sharma A. India as the heritage of medicinal plant and their use. Curr Trends Biomedical Eng & Biosci 2017; 4(4): 50-1.]. The parts of medicinal plants that may be used are different types of seeds, root, leaf, fruit, flowers or even the whole plant [6Jamshidi KF, Lorigooini Z, Amini KH. Medicinal plant: Past history and future perspective. J Herbmed Pharmacol 2018; 7(1): 1-7.
[http://dx.doi.org/10.15171/jhp.2018.01] ].
Tinospora cordifolia (Wild.) Hook.f. & Thomson commonly known as guduchi, heart-leaved moonseed and giloya, indigenous to the tropical areas of India, Myanmar and Sri Lanka, is a herbaceous vine of the family Menispermaceae. Guduchi is widely used in veterinary folk medicine/ ayurvedic system of medicine for its genera tonic, antiperiodic, antispasmodic, anti-inflammatory, anti-arthritic, anti-allergic and anti-diabetic properties [7Spandhan U, Shaik LA, Nirmala T, Santhi M. SD Sipai B. A review on Tinospora cordifolia. Int J Cur Pharma Rev Res 2013; 4(2): 61-8., 8Zhao TF, Wang X, Rimando AM. Che. Folkloric medicinal plants: Tinospora sagittatavar. cravaniana and Mahonia bealei. Planta Med 1991; 57: 507.
[http://dx.doi.org/10.1055/s-2006-960188] ]. Tinospora cordifolia is a rich source of alkaloids, furan diterpenoids, clerodane, norditerpenoids, sequiterpenoids, phenolics, lignans, sterols, aliphatic compounds, polysaccharides, essential oil and fatty acids. In its pharmacological actions, Tinospora cordifolia targets body organs, mainly kidney, liver, and spleen [9Singh D, Chaudhuri PK. Chemistry and pharamacology of Tinospora cordifolia. Nat Prod Commun 2017; 12(2): 299-308.
[http://dx.doi.org/10.1177/1934578X1701200240] [PMID: 30428235] ]. The genus Tinospora cordifolia has been widely investigated by a number of workers and reported to contain a number of phytochemicals with marked therapeutic activity [10Premanath R, Lakshmi D. Studies on antioxidant activity of Tinospora cordifolia leaves using in-vitro models. J Am Sci 2010; 6(10): 736-43.-26Zeba S, Mishra PK. Phytochemical study of Tinospora cordifolia grown on three different soil conditions. Res J Life Sci, Bioinf Pharma Chem Sci 2019; 5(2): 810-5.]. Free radicals form in our body as a result of biological oxidation. Oxidation is a natural process in organisms for the production of energy to fuel biological cycles. Oxidation by-products of normal metabolism cause extensive damage to DNA, protein and lipids, constituting a major contribution to ageing and also to degenerative disease. Oxidative damage is associated with chronic degenerative diseases, including cancer, coronary artery disease, hypertension and diabetes [14Upadhyay N, Ahmad G, Agnihotri K, Sharma R. Studies on antioxidant activity and total phenolic content of Tinospora cordifolia stem using in vitro models. Am J Phytomed Clinical Therapeutics 2013; 8: 617-27.]. An antioxidant is a chemical that prevents the oxidation of other chemicals. They protect the key cell components by neutralizing the damaging effects of free radicals, which are natural by-products of cell metabolism [27Miller HE, Rigelhof F, Marquart L, Prakash A, Kanter M. Antioxidant content of whole grain breakfast cereals, fruits and vegetables. J Am Coll Nutr 2000; 19(3)(Suppl.): 312S-9S.
[http://dx.doi.org/10.1080/07315724.2000.10718966] [PMID: 10875603] ]. Antioxidants occur naturally in many fruits and are able to neutralize free radicals by donating an electron and convert them into harmless molecules [28Markis DP, Boskou G, Andrikopoulos NK. Polyphenolic content and in vitro antioxidant charactristics of wine industry and other agri-food solid waste extracts. J Food Compos Anal 2007; 20(2): 125-32.
[http://dx.doi.org/10.1016/j.jfca.2006.04.010] ].
During the last few decades, there has been an increasing interest of scientists in the study of medicinal plants and surveys on their traditional use all over the world. Although some eminent work on Tinospora cordifolia has been reported from some areas of the world like Angat, Bulacan, Philippines [16Castillo AL, Ysrael MC, Cirna JCD, et al. Free radical scavenging activity of Tinospora cordifolia stem methanolic fraction. Int J Biol Pharm Allied Sci 2015; 4(10): 6241-54.] and some from different regions of India like Mysore [10Premanath R, Lakshmi D. Studies on antioxidant activity of Tinospora cordifolia leaves using in-vitro models. J Am Sci 2010; 6(10): 736-43., 11IIaiyaraja N, Khanum F. Antioxidant potential of Tinospora cordifolia extracts and their protective effects on oxidation of biomolecules. Pharmacogn J 2011; 3(20): 56-61.
[http://dx.doi.org/10.5530/pj.2011.20.11] ], Ranchi [13Kumar A, Kumar M, Dandapat S, Sinha MP. Antioxidant activity and pharmacological screening of Tinospora cordifolia (Thunb.). Biosean 2013; 8(2): 689-93.], Khandari Campus Agra [14Upadhyay N, Ahmad G, Agnihotri K, Sharma R. Studies on antioxidant activity and total phenolic content of Tinospora cordifolia stem using in vitro models. Am J Phytomed Clinical Therapeutics 2013; 8: 617-27., 15Upadhyay N, Showkat A, Ahmad G, Agnihotri RK, Sharma R. Free radical scavenging activity of Tinospora cordifolia (Willd) Miers. J Pharmacogn Phytochem 2014; 3(2): 63-9.], Bengaluru [18Shwetha RJ. Tahareen, Myrene RD. Antioxidant and anti-inflammatory activity of Tinospora cordifolia using in vitro models. J Chem Biol Phys Sci 2016; 6(2): 497-512.], Gwalior (MP) [19Kumar V, Singh S, Singh A, Kumar DA. Srivastava, Sidhu G, Singh R, Meena, Singh R, Subhose Varanasi, Prakash O. Phtochemical, antioxidant, antimicrobial and protein binding qualities of hydro-ethanolic extract of Tinospora cordifolia. J Bio Active Products from Nature 2018; 8(3): 192-200.
[http://dx.doi.org/10.1080/22311866.2018.1485513] ], Udupi (Karnataka) [20Polu RP, Nayanbhirama U, Khan S, Rajlexmi M. Assessment of free radical Scavenging and anti-proliferative activites of Tinospora cordifolia. BMC Complement Altern Med 2017; 17: 2-12.
[http://dx.doi.org/10.1186/s12906-017-1953-3] ], Chitrakoot region (MP) [22Garg P, Grag R. Qualitative and quantitative of leaves and stem of Tinospora cordifolia in different solvent extract. J Drug Deliv Ther 2018; 8(5): 259-64.
[http://dx.doi.org/10.22270/jddt.v8i5-s.1967] ], West Singhkhum (Jharkhand) [23Sinku R, Sinha MR. Preliminary phytochemical screening and physiochemical analysis of Tinospora cordifolia. Miers J Medl plant Stu 2018; 6(1): 177-80.], Hazaribagh, and Bokaro (Jharkhand) [24Prasad B, Chauhan A. Antioxidant and antimicrobial studies of Tinospora cordifolia stem and roots under in Vitro conditions. Int J Adv Micro Health Res 2019; 3(1): 1-10.]. However, comparative reports on Himalayan Tinospora cordifolia stem and leaf extracts so far have not been investigated. Therefore, keeping these points in mind, the present study was undertaken in order to identify the antioxidant potential and quantitative analysis of stem and leaf extracts of Tinospora cordifolia (Wild.) Hook.f. & Thomson, which were procured from the Haldwani regions of Kumaun Himalaya.
2. MATERIALS AND METHODS
2.1. Collection and Processing of Plant Materials
The plant material (Tinospora cordifolia (Wild.) Hook.f. & Thomson) of the present study was collected from Haldwani, District Nainital, Uttarakhand, India (Latitude: 29.17558; Longitude: 79.51718; Altitude: 424m). The identity of the collected specimen was authenticated and submitted at the Taxonomy lab of D. S. B. Campus, Nainital. The plant material used for antioxidant activity was thoroughly washed off under running tap water to remove all the debris and soil and shade dried at room temperature for two to three weeks. The air-dried plant material was finely ground into powdered and packed in self-seal airtight polythene bags for further analysis [14Upadhyay N, Ahmad G, Agnihotri K, Sharma R. Studies on antioxidant activity and total phenolic content of Tinospora cordifolia stem using in vitro models. Am J Phytomed Clinical Therapeutics 2013; 8: 617-27.].
2.2. Extraction
Fivegram powder of each stem and leaf of Tinospora cordifolia (Wild.) Hook.f. & Thomson was soaked in 50 mL of methanol solvent in an orbital shaker at 37°C 120 rpm for 3 days. After that, the extracts were filtered with the help of Waltman No. 1 and solvent was evaporated in an incubator at 37°C to get the syrupy consistency, then 25% DMSO (Dimethyl sulphoxide) was added and after that the extracts were kept in a refrigerator at 0°C to determine the antioxidant activity [14Upadhyay N, Ahmad G, Agnihotri K, Sharma R. Studies on antioxidant activity and total phenolic content of Tinospora cordifolia stem using in vitro models. Am J Phytomed Clinical Therapeutics 2013; 8: 617-27.].
2.3. Antioxidant Activity Measurement
The scavenging activity of Tinospora cordifolia (Wild.) Hook.f. & Thomson stem and leaf extracts was determined using DPPH and ABTS assay.
2.3.1. DPPH (1,1-Diphenyl -2-picrylhydrazyl) free radical scavenging assay
The method adopted for this study was developed by Blois (1958), which depends on the reduction of purple DPPH to yellow coloured diphenyl picrylhydrazine radical scavenger [29Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958; 181: 1199-200.
[http://dx.doi.org/10.1038/1811199a0] ]. A solution of the radical was prepared by dissolving 1mg/m L DPPH in methanol. The determination of the disappearance of the free radical was done using a UV-visible spectrophotometer. Each plant extract sample stock solution (1mg/mL) was diluted in final concentration of 10, 20, 40, 80, 160, 320, 640µg/mL). Ascorbic acid was used as positive control and prepared in the same manner as an extract. As DPPH is sensitive to light, it is exposed to minimum sensitive light. These solutions were allowed to react at room temperature for 30 minutes in the dark [14Upadhyay N, Ahmad G, Agnihotri K, Sharma R. Studies on antioxidant activity and total phenolic content of Tinospora cordifolia stem using in vitro models. Am J Phytomed Clinical Therapeutics 2013; 8: 617-27.].
The absorbance values were measured at 517nm and converted into percentage antioxidant activity using the following equation:
(where Abs =Absorbance)
All the determinations were performed in triplicate.
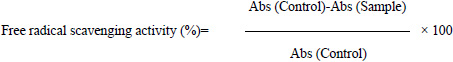 |
2.3.2. ABTS (2,2´-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) Free Radical Scavenging Assay
This method depends on the reduction of sea green coloured ABTS to transparent ABTS [30Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med 2012; 12(1): 221.
[http://dx.doi.org/10.1186/1472-6882-12-221] [PMID: 23153304] ]. ABTS + cation radical was produced by a reaction between 7mM ABTS and 2.45mM potassium persulfate stored in the dark at room temperature for overnight before use. The solution was diluted with 50% methanol. Each plant extract sample stock solution (1mg/mL) was diluted in a final concentration of 10, 20, 40, 80, 160, 320, 640µg/mL). Ascorbic acid was used as positive control and prepared in the same manner as above. Then 2mM ABTS added to different concentrations of extract and incubate in the dark for 30 min at room temperature. As ABTS is sensitive to light, it is exposed to minimum sensitive light at room temperature. The determination of the disappearance of the free radical was done using a UV-visible spectrophotometer. All the measurements were carried out at least three times [20Polu RP, Nayanbhirama U, Khan S, Rajlexmi M. Assessment of free radical Scavenging and anti-proliferative activites of Tinospora cordifolia. BMC Complement Altern Med 2017; 17: 2-12.
[http://dx.doi.org/10.1186/s12906-017-1953-3] ]. Percent inhibition of absorbance at 745nm was calculated using the formula:
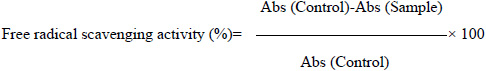 |
(where Abs =Absorbance)
All the determinations were performed in triplicate.
The concentration of any antioxidant, which shows 50% inhibition, is called as IC50 value. In the experiment, IC50 values for extracts and standards were calculated from the regression plot between serial dilutions and the % inhibition.
2.4. Quantitative Analysis
2.4.1. Total Phenolic Content
Total phenolics were estimated by following Folin-Ciocalteu's phenol reagent (FCR) method of Upadhyay et al. with little modification [14Upadhyay N, Ahmad G, Agnihotri K, Sharma R. Studies on antioxidant activity and total phenolic content of Tinospora cordifolia stem using in vitro models. Am J Phytomed Clinical Therapeutics 2013; 8: 617-27.]. Different concentrations (0.5,1, 2, 4, 8mg/mL) of standard and test samples were prepared in methanol from the stock solution. 0.5mL of each concentration was mixed with 0.2 mL of FCR and 0.5 mL Na2CO3 (20%). After 30min of incubation at room temperature, absorbance was measured at 750nm against blank (methanol). The concentration of total phenolic content was calculated as mg gallic acid equivalent/g extract from the calibration curve of the standard solution of gallic acid.
2.4.2. Total Flavonoid Content
Total flavonoid content was estimated by aluminium chloride (AlCl3) method. Different concentrations (0.5, 1, 2, 4, 8mg/mL) of standard and test samples were prepared in methanol from the stock solution. 0.5ml of each concentration was mixed with 0.2 ml of AlCl3, 0.5mL NaNo3 (20%). The concentration of total flavonoid content was calculated as mg quercetin acid equivalent/g extract, from the calibration curve of the standard solution of quercetin acid. Then allow the solution at room temperature for 30 min. The absorbance was measured at 506nm by spectrophotometer [22Garg P, Grag R. Qualitative and quantitative of leaves and stem of Tinospora cordifolia in different solvent extract. J Drug Deliv Ther 2018; 8(5): 259-64.
[http://dx.doi.org/10.22270/jddt.v8i5-s.1967] ].
3. RESULTS AND DISCUSSION
3.1. Antioxidant Activity
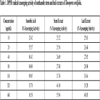
3.1.1. DPPH (1,1-Diphenyl-2-picrylhydrazyl) Free Radical Scavenging Activity of Stem and Leaf Extracts
Both the stem and leaf extracts of Tinospora cordifolia (Wild.) Hook.f. & Thomson showed effective scavenging activity against the free radicals. The DPPH standard (ascorbic acid) had a maximum (96.58%) free radical scavenging activity at 640µg/mL and with the lowest IC50 value of 22.38 µg/mL (Tables 1-3). The methanolic stem extract showed the highest scavenging activity (55.94%) at 640µg/mL and lowest (25.32%) at 10µg/mL (Table 1). It was found that when the concentration of the extract increased, the absorbance value gets decreased regularly. The methanolic leaf extract of T. cordifolia showed comparatively lesser activity with the highest scavenging activity (48.03%) at 640 µg/mL and the lowest (25.01%) at 10µg/mL (Table 1) as compared to the methanolic stem extract. Similar results were also observed by Upadhyay et al. [14Upadhyay N, Ahmad G, Agnihotri K, Sharma R. Studies on antioxidant activity and total phenolic content of Tinospora cordifolia stem using in vitro models. Am J Phytomed Clinical Therapeutics 2013; 8: 617-27.] in the methanolic stem extract of T. cordifolia and found that at a concentration of 10 mg/mL, DPPH free radical scavenging activity was low (44%). In contrast, Praveen et al. [31Praveen N, Thiruvengadam M, Kim HJ, Kumar JKP, Chung IM. Antioxidant activity of Tinospora cordifolia leaf extract through non-enzymatic method. J Med Plants Res 2012; 6(3): 4790-5.] reported that methanolic leaf extract showed the highest scavenging activity. Scavenging effect on DPPH radical was observed to be higher in methanolic leaf extract as compared to the methanolic extract of stem of T. cordifolia procured from the local market of Mysore [11IIaiyaraja N, Khanum F. Antioxidant potential of Tinospora cordifolia extracts and their protective effects on oxidation of biomolecules. Pharmacogn J 2011; 3(20): 56-61.
[http://dx.doi.org/10.5530/pj.2011.20.11] ]. The leaf and stem extracts of the methanolic solvent of T. cordifolia showed greater percentage inhibition with IC50 values of 0.54 mg/mL and IC50 0.74 mg/mL, respectively, which was more than the present study [11IIaiyaraja N, Khanum F. Antioxidant potential of Tinospora cordifolia extracts and their protective effects on oxidation of biomolecules. Pharmacogn J 2011; 3(20): 56-61.
[http://dx.doi.org/10.5530/pj.2011.20.11] ]. In a previous study, the methanolic stem extract of T. cordifolia from the Philippines exhibited good free radical scavenging activity potential with an IC50 value of 0.861 µg/µL [16Castillo AL, Ysrael MC, Cirna JCD, et al. Free radical scavenging activity of Tinospora cordifolia stem methanolic fraction. Int J Biol Pharm Allied Sci 2015; 4(10): 6241-54.].
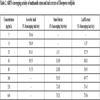
3.1.2. ABTS (2,2´-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) Free Radical Scavenging Activity of Stem and Leaf Extracts
All the concentration of the test solution more or less scavenged the free radicals. Both the stem and leaf extracts of Tinospora cordifolia showed effective scavenging activity against ABTS free radical. The ABTS standard (Ascorbic acid) had maximum free radical scavenging activity (91.55%) at 80µg/mL concentration with the least IC50 value of 9.36 µg/mL as represented in Table 2. The methanolic stem extract showed the highest scavenging activity (74.21%) at 640µg/mL concentration and the lowest (4.51%) at 20µg/mL (Table 2). The methanolic leaf extract of T. cordifolia showed comparatively lesser activity with the highest scavenging activity (73.86%) at 640 µg/mL concentration and the lowest (6.33%) at 10µg/mL concentration as compared to the methanolic stem extract (Table 2). The stem extract of T. cordifolia showed better IC50 value in ABTS (126.93 µg/mL) and DPPH (500.40 µg/mL) assays as compared to the leaf extract of the plant with IC50 values if 379.31 µg/mL and 677.78µg/mL in ABTS and DPPH assays respectively (Table 3). With all the extracts (leaf and stem), dose-dependent radical scavenging was observed, i.e., when the concentration of the extract was increased, the free radical scavenging activity also increased. These results are in agreement with reports of Upadhyay et al. [14Upadhyay N, Ahmad G, Agnihotri K, Sharma R. Studies on antioxidant activity and total phenolic content of Tinospora cordifolia stem using in vitro models. Am J Phytomed Clinical Therapeutics 2013; 8: 617-27.]. ABTS scavenging assay of leaf and stem extracts of the methanolic solvent of T. cordifolia from Mysore showed greater inhibition as compared to the present study with the least IC50 values of 95µg/mL and 107 µg/mL [11IIaiyaraja N, Khanum F. Antioxidant potential of Tinospora cordifolia extracts and their protective effects on oxidation of biomolecules. Pharmacogn J 2011; 3(20): 56-61.
[http://dx.doi.org/10.5530/pj.2011.20.11] ]. The ABTS scavenging assay of both leaf and stem extracts was the most potent method as compared to the DPPH scavenging assay with the highest scavenging activity and least IC50 values.
3.2. Quantitative Analysis
3.2.1. Total Phenolic Content (TPC)
TPC was measured by folin-ciocalteu's phenol reagent method and gallic acid was taken as standard in different concentrations (mg/mL). TPC of methanol extract of Tinospora cordifolia leaves and stem was calculated as gallic acid equivalent (GAE) (mg GAE/g), using the calibration curve, which was based on the following equation: Y= 1.5118x-0.1533 R2=0.9664 shown in Fig. (1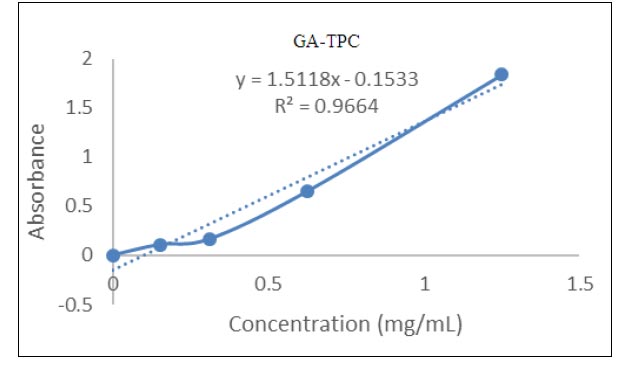 ). It was clear that the phenolic content in methanolic leaf extract (8.51 mg GAE /g) was lower as compared to the methanolic stem extract (17.44 mg GAE /g) of Tinospora cordifolia as shown in Table 4. The assay supported that there was a positive relationship between total phenolic content and antioxidant activity. The TPC of methanolic stem extract was less than the TPC in the methanolic stem extract (51.86 mg GAE /g), as reported by Upadhyay et al. [14Upadhyay N, Ahmad G, Agnihotri K, Sharma R. Studies on antioxidant activity and total phenolic content of Tinospora cordifolia stem using in vitro models. Am J Phytomed Clinical Therapeutics 2013; 8: 617-27.]. Similar results were reported by IIaiyaraja and Khanum [11IIaiyaraja N, Khanum F. Antioxidant potential of Tinospora cordifolia extracts and their protective effects on oxidation of biomolecules. Pharmacogn J 2011; 3(20): 56-61.
). It was clear that the phenolic content in methanolic leaf extract (8.51 mg GAE /g) was lower as compared to the methanolic stem extract (17.44 mg GAE /g) of Tinospora cordifolia as shown in Table 4. The assay supported that there was a positive relationship between total phenolic content and antioxidant activity. The TPC of methanolic stem extract was less than the TPC in the methanolic stem extract (51.86 mg GAE /g), as reported by Upadhyay et al. [14Upadhyay N, Ahmad G, Agnihotri K, Sharma R. Studies on antioxidant activity and total phenolic content of Tinospora cordifolia stem using in vitro models. Am J Phytomed Clinical Therapeutics 2013; 8: 617-27.]. Similar results were reported by IIaiyaraja and Khanum [11IIaiyaraja N, Khanum F. Antioxidant potential of Tinospora cordifolia extracts and their protective effects on oxidation of biomolecules. Pharmacogn J 2011; 3(20): 56-61.
[http://dx.doi.org/10.5530/pj.2011.20.11] ] with total phenolic content of 33.93 mg GAE /g and 52.17 mg GAE /g in the methanolic stem and methanolic leaf extracts of T, cordifolia, respectively. The total phenolic content of methanolic leaf extract of T. crodifolia was reported to be 2.00 mg GAE /g by Shwetha et al. [18Shwetha RJ. Tahareen, Myrene RD. Antioxidant and anti-inflammatory activity of Tinospora cordifolia using in vitro models. J Chem Biol Phys Sci 2016; 6(2): 497-512.], which was less than the present findings.
 |
Fig. (1) Standard curve of gallic acid for total phenolic content. |
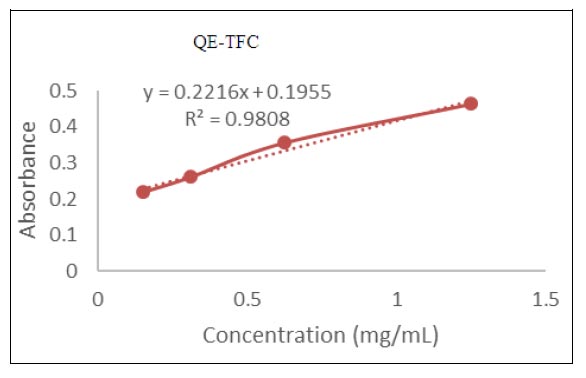 |
Fig. (2) Standard curve of quercetin for total flavonoid content. |
3.2.2. Total Flavonoid Content (TFC)
The TFC was measured by aluminium chloride reagent method and quercetin was taken as standard. TFC of methanol extract of Tinospora cordifolia leaves and stem extracts was calculated as Quercetin Equivalent (QE) (mg/g), using a calibration curve, which was based on the following equation: Y = 0.2216x+ 0.1955 R2=0.9808 as shown in Fig. (2 ). The flavonoid content of the methanolic leaf extract (20.94 mg QE/g) was lower as compared to the methanolic stem extract (23.82 mg QE/g) of T. cordifolia as shown in Table 4. The high flavonoid content was found to be positively correlated with the antioxidant activity. The TFC in the methanolic leaf extract of T. cordifolia was 0.7 mg QE/g reported by Shwetha et al. [18Shwetha RJ. Tahareen, Myrene RD. Antioxidant and anti-inflammatory activity of Tinospora cordifolia using in vitro models. J Chem Biol Phys Sci 2016; 6(2): 497-512.], which was less than the present findings. The methanolic stem extract showed higher antioxidant activity and higher phenol and flavonoid content as compared to the Methanolic leaf extract. The similar results were observed by Garg and Garg [22Garg P, Grag R. Qualitative and quantitative of leaves and stem of Tinospora cordifolia in different solvent extract. J Drug Deliv Ther 2018; 8(5): 259-64.
). The flavonoid content of the methanolic leaf extract (20.94 mg QE/g) was lower as compared to the methanolic stem extract (23.82 mg QE/g) of T. cordifolia as shown in Table 4. The high flavonoid content was found to be positively correlated with the antioxidant activity. The TFC in the methanolic leaf extract of T. cordifolia was 0.7 mg QE/g reported by Shwetha et al. [18Shwetha RJ. Tahareen, Myrene RD. Antioxidant and anti-inflammatory activity of Tinospora cordifolia using in vitro models. J Chem Biol Phys Sci 2016; 6(2): 497-512.], which was less than the present findings. The methanolic stem extract showed higher antioxidant activity and higher phenol and flavonoid content as compared to the Methanolic leaf extract. The similar results were observed by Garg and Garg [22Garg P, Grag R. Qualitative and quantitative of leaves and stem of Tinospora cordifolia in different solvent extract. J Drug Deliv Ther 2018; 8(5): 259-64.
[http://dx.doi.org/10.22270/jddt.v8i5-s.1967] ] showing lesser flavonoid content in methanolic stem extract (2.14 mg QE/g) as compared to the methanolic leaf extract (4.53 mg QE/g).
CONCLUSION
The plant kingdom is a treasure of potential drugs and the drugs from the plants are easily available, less expensive, safe, efficient and rarely have side effects. In the present study, Tinospora cordifolia (Wild.) Hook.f. & Thomson, which was collected from Haldwani (Nainital), was assessed for its antioxidant activity and quantitative analysis. The stem extract exhibited more antioxidant activity than the leaf extract with regards to the all parameters analyzed.
ETHICAL STATEMENT
The plant material (Tinospora cordifolia (Wild.) Hook.f. & Thomson) of the present study was collected from Haldwani, District Nainital, Uttarakhand, India (Latitude: 29.17558; Longitude: 79.51718; Altitude: 424m). The identity of the collected specimen was authenticated and submitted at the Taxonomy lab of D. S. B. Campus, Nainital.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are grateful to the Head, Department of Botany, D. S. B. Campus, Nainital, for necessary laboratory facilities.
REFERENCES
| [1] | Nadkarni KM. India material medica 1976. |
| [2] | Dastur JF. Medicinal plants of India and Pakistan 1985. |
| [3] | Saradamma I. All India co-ordinated research project on ethanobiology Final technique report RRI Drug Research, CCRAS, Government of India, Poojapura 1990. |
| [4] | Mittal J, Sharma MM, Batra A. Tinospora cordifolia a multipurpose medicinal plant- A review. J Med Plants Stud 2014; 2: 32-47. |
| [5] | Gangola S, Khati P. Parul, Bhatt P, Sharma A. India as the heritage of medicinal plant and their use. Curr Trends Biomedical Eng & Biosci 2017; 4(4): 50-1. |
| [6] | Jamshidi KF, Lorigooini Z, Amini KH. Medicinal plant: Past history and future perspective. J Herbmed Pharmacol 2018; 7(1): 1-7. [http://dx.doi.org/10.15171/jhp.2018.01] |
| [7] | Spandhan U, Shaik LA, Nirmala T, Santhi M. SD Sipai B. A review on Tinospora cordifolia. Int J Cur Pharma Rev Res 2013; 4(2): 61-8. |
| [8] | Zhao TF, Wang X, Rimando AM. Che. Folkloric medicinal plants: Tinospora sagittatavar. cravaniana and Mahonia bealei. Planta Med 1991; 57: 507. [http://dx.doi.org/10.1055/s-2006-960188] |
| [9] | Singh D, Chaudhuri PK. Chemistry and pharamacology of Tinospora cordifolia. Nat Prod Commun 2017; 12(2): 299-308. [http://dx.doi.org/10.1177/1934578X1701200240] [PMID: 30428235] |
| [10] | Premanath R, Lakshmi D. Studies on antioxidant activity of Tinospora cordifolia leaves using in-vitro models. J Am Sci 2010; 6(10): 736-43. |
| [11] | IIaiyaraja N, Khanum F. Antioxidant potential of Tinospora cordifolia extracts and their protective effects on oxidation of biomolecules. Pharmacogn J 2011; 3(20): 56-61. [http://dx.doi.org/10.5530/pj.2011.20.11] |
| [12] | Bhalerao BM, Kasote DM, Nagarkar BF, et al. Comparative analysis of radical scavenging and immunomodulatory activities of Tinospora cordifolia growing with different supporting trees. Acta Biol Szeged 2012; 56(1): 65-71. |
| [13] | Kumar A, Kumar M, Dandapat S, Sinha MP. Antioxidant activity and pharmacological screening of Tinospora cordifolia (Thunb.). Biosean 2013; 8(2): 689-93. |
| [14] | Upadhyay N, Ahmad G, Agnihotri K, Sharma R. Studies on antioxidant activity and total phenolic content of Tinospora cordifolia stem using in vitro models. Am J Phytomed Clinical Therapeutics 2013; 8: 617-27. |
| [15] | Upadhyay N, Showkat A, Ahmad G, Agnihotri RK, Sharma R. Free radical scavenging activity of Tinospora cordifolia (Willd) Miers. J Pharmacogn Phytochem 2014; 3(2): 63-9. |
| [16] | Castillo AL, Ysrael MC, Cirna JCD, et al. Free radical scavenging activity of Tinospora cordifolia stem methanolic fraction. Int J Biol Pharm Allied Sci 2015; 4(10): 6241-54. |
| [17] | Dwivedi SK. Enespa. Tinospora cordifolia with reference to Biological and Microbial Properties. Int J Curr Microbiol Appl Sci 2016; 5(6): 446-65. [http://dx.doi.org/10.20546/ijcmas.2016.506.052] |
| [18] | Shwetha RJ. Tahareen, Myrene RD. Antioxidant and anti-inflammatory activity of Tinospora cordifolia using in vitro models. J Chem Biol Phys Sci 2016; 6(2): 497-512. |
| [19] | Kumar V, Singh S, Singh A, Kumar DA. Srivastava, Sidhu G, Singh R, Meena, Singh R, Subhose Varanasi, Prakash O. Phtochemical, antioxidant, antimicrobial and protein binding qualities of hydro-ethanolic extract of Tinospora cordifolia. J Bio Active Products from Nature 2018; 8(3): 192-200. [http://dx.doi.org/10.1080/22311866.2018.1485513] |
| [20] | Polu RP, Nayanbhirama U, Khan S, Rajlexmi M. Assessment of free radical Scavenging and anti-proliferative activites of Tinospora cordifolia. BMC Complement Altern Med 2017; 17: 2-12. [http://dx.doi.org/10.1186/s12906-017-1953-3] |
| [21] | Bharathi C, Harinatha R, Nageswari G. A review of medicinal properties of Tinospora cordifolia. Int J Sci Res Rev 2018; 7: 585-98. |
| [22] | Garg P, Grag R. Qualitative and quantitative of leaves and stem of Tinospora cordifolia in different solvent extract. J Drug Deliv Ther 2018; 8(5): 259-64. [http://dx.doi.org/10.22270/jddt.v8i5-s.1967] |
| [23] | Sinku R, Sinha MR. Preliminary phytochemical screening and physiochemical analysis of Tinospora cordifolia. Miers J Medl plant Stu 2018; 6(1): 177-80. |
| [24] | Prasad B, Chauhan A. Antioxidant and antimicrobial studies of Tinospora cordifolia stem and roots under in Vitro conditions. Int J Adv Micro Health Res 2019; 3(1): 1-10. |
| [25] | Sharma P, Dwivedee BP, Bisht D, Dash AK, Kumar D. The chemical constituents and diverse pharmacological importance of Tinospora cordifolia. Heliyon 2019; 1-8. [http://dx.doi.org/10.1016/j.heliyon.2019.e02437] [PMID: 31701036] |
| [26] | Zeba S, Mishra PK. Phytochemical study of Tinospora cordifolia grown on three different soil conditions. Res J Life Sci, Bioinf Pharma Chem Sci 2019; 5(2): 810-5. |
| [27] | Miller HE, Rigelhof F, Marquart L, Prakash A, Kanter M. Antioxidant content of whole grain breakfast cereals, fruits and vegetables. J Am Coll Nutr 2000; 19(3)(Suppl.): 312S-9S. [http://dx.doi.org/10.1080/07315724.2000.10718966] [PMID: 10875603] |
| [28] | Markis DP, Boskou G, Andrikopoulos NK. Polyphenolic content and in vitro antioxidant charactristics of wine industry and other agri-food solid waste extracts. J Food Compos Anal 2007; 20(2): 125-32. [http://dx.doi.org/10.1016/j.jfca.2006.04.010] |
| [29] | Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958; 181: 1199-200. [http://dx.doi.org/10.1038/1811199a0] |
| [30] | Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med 2012; 12(1): 221. [http://dx.doi.org/10.1186/1472-6882-12-221] [PMID: 23153304] |
| [31] | Praveen N, Thiruvengadam M, Kim HJ, Kumar JKP, Chung IM. Antioxidant activity of Tinospora cordifolia leaf extract through non-enzymatic method. J Med Plants Res 2012; 6(3): 4790-5. |