- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Biomaterials Journal
(Discontinued)
ISSN: 1876-5025 ― Volume 5, 2014
AC Electric Conductivity and Biochemical Analyses of Physiologic Solutions to Follow Biomimetic Coatings on Corals Impregnated with Ag or Zn or Sr Ions
W.I. Abdel-Fattah*, 1 , A.M. Sallam2, I.H. Ibrahim2, H. Ibrahim2
Abstract
The impregnation of sodium hypochlorite (NaOCl) treated corals (Favia Stelligera) from Red Sea coast, Egypt with two moles of either divalent (Sr2+, Zn2+) or mono valent (Ag+) ions for 6 hours at ambient conditions was performed. Subsequent effect on hydroxyapatite (HA) deposition upon immersion in saline and simulated body fluid (SBF) solutions up to 72 h was followed through measuring room temperature Ac-electrical conductivities solutions during immersion periods. The changes in the levels of Calcium Ca2+and inorganic phosphorous iP in SBF were followed by spectrophotometer post 72 h immersion using biochemical kits in both solutions. The microstructures of the impregnated corals were examined using Scanning electron microscope (SEM) and Energy dispersive X-ray analysis (EDX) compared to the control treated with NaOCl.
Various levels of Ca2+ and iP in SBF were obtained in the trend of more HA deposition. Massive Carbonated hydroxyapatite (CHA) formation was achieved proving biolayer formation. The samples possess intermediate porosity of 17% with interconnectivity and architectures similar to bone. It was found that the immersion of impregnated samples in SBF formed HA which differed according to the valency of the ions and its effect on morphology.
Therefore, the samples are suggested as hard tissue engineered constructs that possess antimicrobial effect and/or bone seeking function to reduce implant infection and enhance bone formation.
Article Information
Identifiers and Pagination:
Year: 2009Volume: 1
First Page: 1
Last Page: 9
Publisher Id: TOBIOMTJ-1-1
DOI: 10.2174/1876502500901010001
Article History:
Received Date: 31/12/2008Revision Received Date: 3/3/2009
Acceptance Date: 13/4/2009
Electronic publication date: 7/9/2009
Collection year: 2009
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Biomaterials Dept. National Research Centre, Dokki, Cairo, Egypt; Tel: 020101407566; Fax: 00202 33370931; E-mail: nrcfifi@yahoo.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 31-12-2008 |
Original Manuscript | AC Electric Conductivity and Biochemical Analyses of Physiologic Solutions to Follow Biomimetic Coatings on Corals Impregnated with Ag or Zn or Sr Ions | |
1. INTRODUCTION
In the year 2010, over 52 million women and men aged 50 and over will be affected by osteoporosis and this number will climb to over 61 million by the year 2020 [1]. Bone grafting is used to promote bone fussing in fully detached fractures, in arthrodesis of joint, and to fill defects or cavities in bone. Grafts involve transplantation of bone from either another part of person’s body (auto graft), from another human patient (allograft), or even an animal (xenograft).
Biomaterials may provide a solution to these problems; an ideal bone substitute should be tolerated by the host tissue without any adverse reaction. It should promote bone formation, have appropriate mechanical strength, be malleable and resorbs after it has fulfilled its function [2]. The purpose of a biomaterial is to replace part or perform function of the body in a safe, reliable, economic and physiologically acceptable manner
Complex forms based on natural structures that can mimic natural scaffolds offer an exciting range of avenues for the construction of a bone analog for tissue engineering taking the advantage of tridimensional constructs to deliver vital cells to the damaged sites [3].
Marine corals act as bioactive natural materials because they are biocompatible, biodegradable and osteoconductive materials. Corals from various sources have been shown to have cell attachment, spread, proliferation and differentiation [4]. Although having strength perse, it is a biodegradable material that bonds tightly to bone without surface apatite layer. On other hand, natural corals provide mechanical interlocking bonding provided by the anchoring effect of the newly formed bone into the surface roughness could be considered as a major factor in bone bonding. Both ß- Tricalcium phosphate (ß-TCP) and natural calcite do not have apatite formation on their surfaces in SBF [5, 6] but despite of this, they bond to living bone. These results may be related to the high resorbability of these materials. In contrast, natural abalone shell forms apatite form on its surface in SBF [7] but does not bond to living bone which might be attributed to antibody reaction to protein in the shell.
It was thought to beneficially modify corals by impregnating them with ions such as Silver (Ag+ )which is reported to induce antibacterial and antimicrobial effect upon substitution with hydroxyapatite ceramics. While Strontium (Sr2+ )and Zinc ( Zn2+ )are proved as bone seeking elements.
Various investigations have been performed to develop antibacterial surface coating. Among them Kim, T. N., used the antimicrobial ceramics based on HA with additions of AgNO3, Cu (NO3)2. 3H2O and Zn (NO3)2. 6H2O. He proved that Ag+ HA exhibited complete inhibition of the growth of E. coli after 20h incubation. In contrast to Ag+ HA, the Cu2+ and Zn2+ HA didn't show any antimicrobial effect in his experiment. This implied that the Ag+ HA plays an important role in the inhibition of the growth of E. coli [8]. Also Abou Neel, E. A. et al. used copper oxide (CuO) – containing phosphate based glass fibers (PGF) for potential uses in wound healing with several concentrations of CuO (0, 1, 5, 10 mole %). They found that the addition of CuO into the glass fibers was effective in reducing the number of bacteria attached to the fiber and fibers with 10%mole CuO were most effective in delivering sufficient amounts of antibacterial Cu2+ to prevent colonization and reduce the number of viable bacteria in the local environment [9]. However, Heidenau, F, made a comparative study about the antibacterial potential of different metal ions Cu2+, Ag+, Co2+, Zn2+, Al3+ and Hg2+with respect to both cytotoxicity (tissue cell toxicity) and antibacterial properties. He reported that the growth inhibition tests proved that Ag+ as well as Zn2+ and Hg2+ ions exhibit very strong cytotoxicity at low concentration but Co2+ showed intermediate mode whereas tissue cells tolerated relatively high concentration of Cu2+ and Al3+. These results are similar for bacteria, except the fact that Co2+ and Zn2+ exhibited no significant antibacterial effect at the investigated concentrations and that Cu2+ ions were significantly more effective against bacteria, staphylococcus epidermis, than toxic for tissue cells. The concentration reported for each ion was (Ag+ = 3. 5×10-3, Zn2+ = 3. 6×10-3, Hg2+ = 4. 2×10-3, Cu2+ = 2. 3×10-1, Co2+ = 3. 4×10-2 and Al3+ = 1. 8×10-2 mmol/L). So copper ions prove most adequate to equip implant surfaces with antibacterial properties without decreasing their biocompatibility in a significant manner [10]. However, none of them followed the consequent effect on the apatite deposition.
In a previous work deprotinated corals were impregnated with Cu2+ in various mole concentrations between 0. 5 to2. 0 and proved that the higher Cu2+ concentration affect the more dense surface biolayer formation [11].
In several reports as early as 1991, it was proposed that the essential requirements for an arjpgicial material to bond to living bone is the formation of bone like apatite on its surface when implanted in the living body, and that this in vivo apatite formation can be reproduced in a simulated body fluid (SBF) with ion concentrations nearly equal to those of human blood plasma [12 ]. This means that the in vivo bone bioactivity of a material can be predicted from the apatite formation on its surface in SBF. Since then, in vitro bone bioactivity of various types of materials have been evaluated through apatite formation in SBF. However, the validity of this method has not been systematically assessed. So to investigate the mechanism of the material/ tissue reaction in vitro examination, with SBF has been developed [13]. In 2003, Conventional SBF with the refined recipe was proposed to the technical Committee ISO/TC150 of International Organization for standardization for in the vitro measurement of apatite forming ability of implant materials and is being discussed by the committee [14].
The aim of the present work is dealing with the impregnation of corals with either of Ag+, Zn2+ or Sr2+ ions in 2 moles concentrations each. The assessment of ions mobility and therefore, biocompatibility of corals in biological solutions was followed through AC electrical conductivity measurements using Conductance Bridge. Spectrophotometer and chemical Kits were used to analyze biological solutions pre and post immersion up to72 h. The results are supposed to reflect ion mobility and subsequent biolayer formation on coral surfaces. The validity of AC electrical conductivity using conductance bridge and ion mobility of saline or SBF as a tool for studying the kinetics of bioactive layer formed compared to spectral analysis using biochemical kits was verified. Moreover, the formation of carbonated hydroxyapatite biolayer and other phosphates on the surface are studied by Scanning Electron microscope equipped with X-ray analysis (SEM and EDX)for the samples post 72 h immersion.
2. EXPERIMENTAL PROCEDURES
2.1. Materials
Coral samples Favia stelliger were collected from Red Sea coast, Hurgada, Egypt. Samples preparation and deproteination with 3% sodium hypochlorite NaOCl and characterization were previously reported [15].
2.2. Impregnating Solutions
Three impregnating solutions of different ions Ag+, Zn2+, and Sr2+with similar concentrations of 2. 00 moles each were prepared by dissolving 33. 8, 29. 0 and 21. 0g of each of silver Nitrate [AgNO3] (Laboratory Rasayan), Zinc Nitrate [Zn (NO3)2. 6H2O] (Laboratory Rasayan) and Strontium Nitrate [Sr (NO3)2] (Laboratory Rasayan) in 50 ml bidistilled water respectively and kept for impregnation experiments.
The Cleaned Coral samples having similar porosity (17%) were impregnated with each specific ion. A ratio 1: 10 wt/vol. was maintained for 6 hours at room temperature. Samples were then removed and dried at 90oC overnight in a dryer. Previous work proved optimum conditions of 17% porosity and 2 moles of Cu2+ ions impregnation for 6 hrs [11].
2.3. Immersion in Physiologic Solutions
2.3.1. Immersion in Saline
A Saline solution was prepared by dissolving 0. 9 gm of Sodium Chloride (NaCl) (ADWIC) in 100 ml bidistilled water [16] and used to follow ionic movement during immersion of ion /impregnated solid corals.
2.3.2. Immersion in SBF
Corrected SBF solution was prepared according to Kokubo [14] by dissolving chemicals in ion exchanged and distilled water buffered at pH=7. 5 at 37oC. The samples were immersed with a ratio of (1: 10) S/L for 72 h at room temperature without agitation or renewal of solutions. Samples were removed from solutions post 72 h, washed with bidistilled water and dried (90oC) over night.
The Saline and SBF solutions left post 72 h immersion were kept for biochemical analysis while the solids were dried and examined by SEM equipped with EDX.
2.3.3. Bioactivity Assessment
2.3.3.a. AC Electrical Conductivity
To predict the mechanism of coral-tissue interaction in vitro, Corals were soaked either in saline or SBF filled in a Conductance Bridge which was used to follow any changes in saline or r-SBF during 72 hours soaking. The variations in conductivity due to the effect of releasing or absorbing ions to/or from the biological solutions were recorded.
2.3.3.b. Biochemical Analysis
i . Calcium and Phosphorous Contents
Subsequent effect of ions impregnation on CHA surface deposition was followed post 72 h immersion in both Saline and SBF solution. The level of ionized Calcium and phosphorous in both saline and SBF post 72 h were measured using biochemical kits (Centronic GmbH-Germany kits) and spectrophotometer (Jenway LTD- U. K.). The levels measured were cumulative as the media were not replaced, so the values determined reflect the changes occurring post 72hrs.
ii . Silver, Zinc and Strontium Ions
Both treated control /coral and impregnated/ coral (1gm each)were dissolved in10 ml of HCl (10% of HCl). The strontium, silver and zinc content were measured using atomic absorption spectrophotometer (Perkin Elmer) The recommended flame used was air-acetylene, oxidine ( USA model 3100).
2.3.3.c. Surface Morphology
The surfaces of the impregnated corals post SBF immersion were examined after coating with gold as an adhesive and conductor. The formation of (CHA) biolayer on the surfaces was verified by scanning electron microscopy with X-ray analysis SEM equipped EDX (Philips XL30).
3. RESULTS AND DISCUSSION
3.1. Electrical Conductivity
3.1.1. Saline
The recorded conductivity values are within the range of 120-170 x10-2ohm-1. cm-1. Coral samples of similar porosities and impregnated with similar ionic concentrations of 2 moles of each of Sr2+, Zn2+ or Ag+ possess increased AC conductivity values during the soaking periods up to 72 h (Fig. 1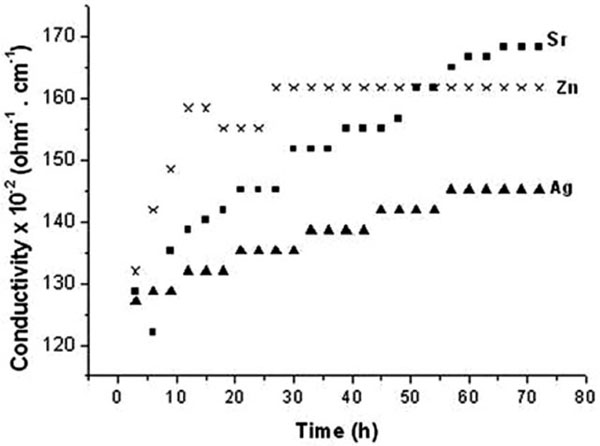 ). Within the initial 10 hrs, sharp increase is recorded for Zn/coral to reach a plateau as early as 26 hrs denoting structure stability. The enhanced values of Zn/coral as early as 12 h are followed by a single plateau as early as 12h and are followed by a single plateau till 72 h denoting structural stability and completion of exchange. The values increase due to ionic release from corals following the order of Zn2+ > Sr2+. >Ag+/coral which continued between 20-55h. At 60 h and above the order changed to follow Sr2+> Zn2+ >Ag+/coral possibly due to exchange with some other ions. On the other hand, Sr2+/and Ag/coral have their conductivity values which exhibit several plataux denoting stepwise increase of conductivity in parallel with released ions from corals.
). Within the initial 10 hrs, sharp increase is recorded for Zn/coral to reach a plateau as early as 26 hrs denoting structure stability. The enhanced values of Zn/coral as early as 12 h are followed by a single plateau as early as 12h and are followed by a single plateau till 72 h denoting structural stability and completion of exchange. The values increase due to ionic release from corals following the order of Zn2+ > Sr2+. >Ag+/coral which continued between 20-55h. At 60 h and above the order changed to follow Sr2+> Zn2+ >Ag+/coral possibly due to exchange with some other ions. On the other hand, Sr2+/and Ag/coral have their conductivity values which exhibit several plataux denoting stepwise increase of conductivity in parallel with released ions from corals.
 |
Fig. (1) The electrical conductivity of saline incubating corals with different ions during 72 hours immersion. |
3.1.2. SBF
The conductivity values are within the range of 0. 5 to 25 x10-2ohm-1. cm-1 which decrease with time and prove, therefore, the ionic deposition on coral surfaces which differed according to ionic species. Such reduced values with time prove ionic deposition from SBF on coral surfaces. Initially the conductivity values of SBF follow the order of Sr2+› Zn2+> Ag+ up to 42 h (Fig. 2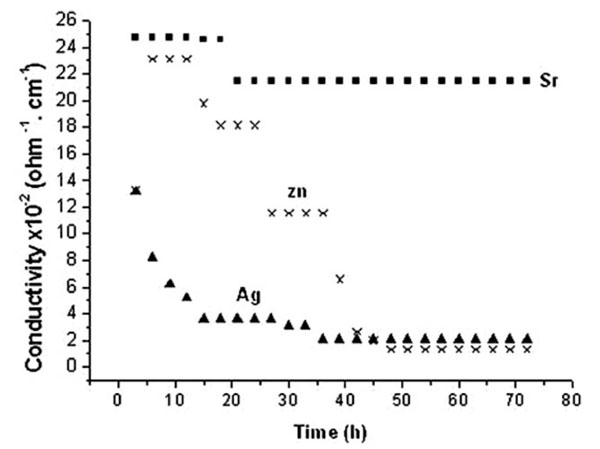 ). As this ionic sequence is similar to saline but in an opposite direction, therefore an involvement in ionic deposition is proved. Measurements at early hours prove that values of Zn/coral are reduced from32 to 2 x10-2ohm-1. cm-1 up to 48 h and above. The stability of ionic exchange is reached by Sr/coral as early as 20 h which is prolonged to 35h for Ag/coral and to 58 h for Zn/coral. The values of conductivity corresponding to Ag/coral are reduced tremendously at 10h to reach one third of its initial value proving ionic deposition. Such trend continued to acquire stability at 35 and above proving completion of deposition.
). As this ionic sequence is similar to saline but in an opposite direction, therefore an involvement in ionic deposition is proved. Measurements at early hours prove that values of Zn/coral are reduced from32 to 2 x10-2ohm-1. cm-1 up to 48 h and above. The stability of ionic exchange is reached by Sr/coral as early as 20 h which is prolonged to 35h for Ag/coral and to 58 h for Zn/coral. The values of conductivity corresponding to Ag/coral are reduced tremendously at 10h to reach one third of its initial value proving ionic deposition. Such trend continued to acquire stability at 35 and above proving completion of deposition.
 |
Fig. (2) The electrical conductivity of SBF incubating corals with different ions during 72 h immersion. |
3.2. Biochemical Measurements
3.2.1. Saline
Analyses of saline from different cation /impregnated corals after 72 h soaking prove the high release of iP from Sr/coral is turned to absorption for Ag/and Zn/coral (Fig. 3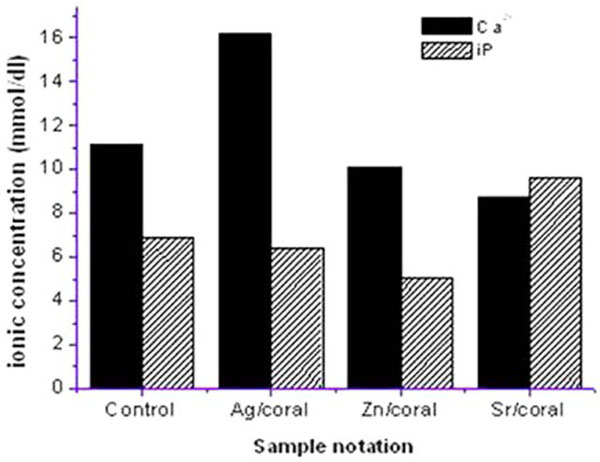 ). A reverse behavior of Ca2+which is highest for Ag /coral followed by Zn/ and Sr /coral with a net result of highest conductivity for Sr/coral. Ionic exchange is to be expected for divalent cations of Sr and Ca between impregnated coral surface and ions in the SBF media consisting of positive and negative ions as well. The order of Ca deposition would be the reverse of its values in SBF following the trend of Sr>Zn>control>Ag which is coinciding with the electric conductivity of the saline solutions containing impregnated corals.
). A reverse behavior of Ca2+which is highest for Ag /coral followed by Zn/ and Sr /coral with a net result of highest conductivity for Sr/coral. Ionic exchange is to be expected for divalent cations of Sr and Ca between impregnated coral surface and ions in the SBF media consisting of positive and negative ions as well. The order of Ca deposition would be the reverse of its values in SBF following the trend of Sr>Zn>control>Ag which is coinciding with the electric conductivity of the saline solutions containing impregnated corals.
 |
Fig. (3) The Concentration of Ca2+, iP in saline post 72 h soaking of coral with 17% porosity. |
3.2.2. SBF
Analyses of SBF left after 72 h incubating the impregnated corals reveal the enhanced values of Ca2+and iP compared to control/coral with an order of Ag>Zn>Sr for Ca 2+while an order of Sr>Ag>Zn is followed for iP.
Interesting is the behaviour of control coral which adsorbs Ca 2+ from SBF and iP to a higher extent which is the due to the positively charged surface of the corals (Fig.4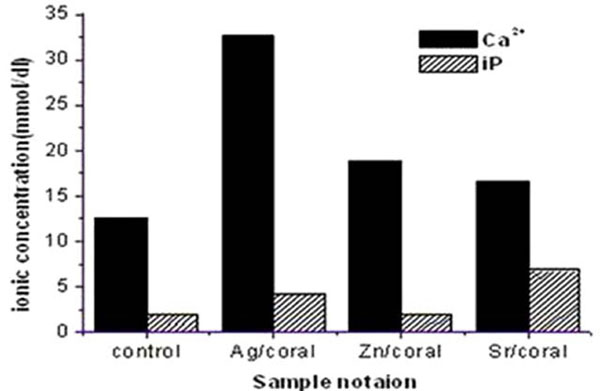 ). These trends are similar to those encountered in saline but the values are nearly doubled. Therefore, all the samples prove release of these ions which are adsorbed on the coral surface to be involved in the surface formed apatitic phases. . The ionic release of Ca 2+ from impregnated corals is nearly double the values recorded for saline proving its higher adsorption at earlier periods of than72 h. On the other hand, iP values being slightly lower are pointing to its adsorption on the coral surfaces due to its positively charged surface.
). These trends are similar to those encountered in saline but the values are nearly doubled. Therefore, all the samples prove release of these ions which are adsorbed on the coral surface to be involved in the surface formed apatitic phases. . The ionic release of Ca 2+ from impregnated corals is nearly double the values recorded for saline proving its higher adsorption at earlier periods of than72 h. On the other hand, iP values being slightly lower are pointing to its adsorption on the coral surfaces due to its positively charged surface.
 |
Fig. (4) The Concentration of Ca2+, iP in SBF post 72 hrs soaking of coral with 17% porosity. |
In Saline as Ca/iP ratios follow an order of Ag>Zn>control corals whose corresponding values are 1. 96>1. 53>1. 25 respectively, being higher than 1, therefore, all are expected to induce transformation of the formed surface amorphous calcium phosphate (ACp)to hydroxyl apatite (HA) (Fig. 5a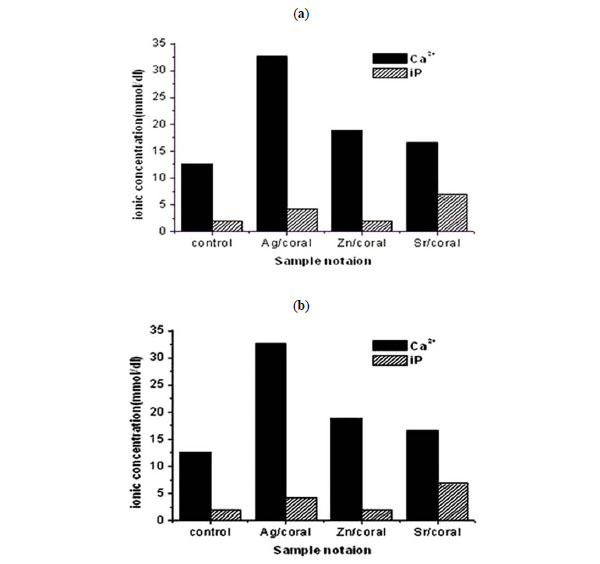 ). However, for Sr/coral having Ca/iP ratio of 0. 87 which is less or equal to 1, the expected phase will be a mixture of HA and brushite [17, 18]
). However, for Sr/coral having Ca/iP ratio of 0. 87 which is less or equal to 1, the expected phase will be a mixture of HA and brushite [17, 18]
 |
Fig. (5) (a) Calcium /phosphorous ratio (Ca/iP) and (b) the activity product [Ca] [iP], of physiological solutions. |
In SBF all the samples released Ca2+and iP ions to SBF post 72 h immersion. The values of Ca/iP ratio are higher than 1, therefore, all phases will transform from amorphous calcium phosphate (ACp) to hydroxyapatite (HA) following the order of Zn> Ag>control>Sr/corals having Ca/iP ratios of 7. 83>6. 23>5. 16>1. 95 respectively.
According to the non-classical nucleation theory [19], at given activity product [Ca2+] [iP], rapid amorphous nucleation occurs at higher Ca/ iP ratio while slow one occurs at lower ratio. Therefore, slowest nucleation of apatitic layer is expected for Sr/Coral whose activity product Ca2+ iP is 1. 95, while highest process is expected for Zn/coral having a value of 7. 83 (Fig. 5b ).
).
Additionally, at high activity product Ca iP nucleation would be homogeneous as for Ag/coral which amounts to 0. 1x10 -6 M 2while heterogeneous direct nucleation could occur as for Zn/coral compared to Sr/Coral having 0. 02 and 0. 08 x10 -6 M 2 respectively.
3.2.3. Morphological Features
Scanning Electron Microscope (SEM) examination of corals skeleton having 17% porosity and impregnated with the same concentration 2 moles of different ions (Ag, Zn and Sr) are shown in (Figs. 6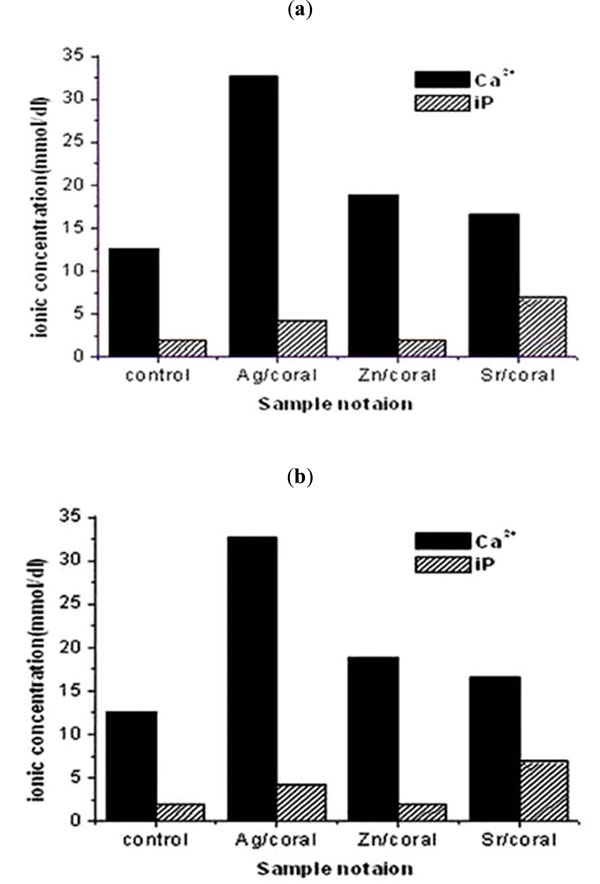 -9
-9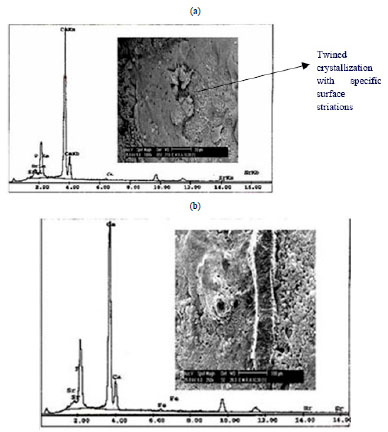 ) respectively.
) respectively.
 |
Fig. (6) The whole skeleton of a)Control impregnated coral, b)Ag/coral, c)Zn/coral, d)Sr/coral. |
At bar width of 2 mm, the whole skeletons of the mentioned samples are shown for each ion (Fig. 6a -d
-d ). At such magnification it is clear that all of them become coated compared to control with several precipitations especially inside the chambers which become nearly closed covering original spines.
). At such magnification it is clear that all of them become coated compared to control with several precipitations especially inside the chambers which become nearly closed covering original spines.
3.2.3.a. Ag / Coral
For Ag / coral sample, there is massive crystallization on the septum of coral chambers. Also there are several types of cubes inside chambers with massive crystallization and lines of depositions. The EDX of this crystallization reveals the presence of Ca, Ag and Cl (Fig. 7a ). At the same magnification, in another area there are several forms of crystallization of cubes and needles (Fig. 7b
). At the same magnification, in another area there are several forms of crystallization of cubes and needles (Fig. 7b ). The EDX of this area reveal the presence of Ca, Ag and Cl while elemental Fe is present as a trace element. The presence of elemental chloride is due to cleaning with NaOCl while the elemental Fe is from the structure of the parent coral. Fig. (7c
). The EDX of this area reveal the presence of Ca, Ag and Cl while elemental Fe is present as a trace element. The presence of elemental chloride is due to cleaning with NaOCl while the elemental Fe is from the structure of the parent coral. Fig. (7c ) shows streaks or leaflets of deposition and is full with cracks due to solution treatment. The EDX of this area reveals the presence of Ca, Ag. P, Fe, Sr and Cl which indicate that not all Ag is released from coral but still present some in phosphate compounds from SBF. Other elements of P, Fe and Sr are representing the original coral. From these results, it is clear that the samples are enriched with Ca and Ag.
) shows streaks or leaflets of deposition and is full with cracks due to solution treatment. The EDX of this area reveals the presence of Ca, Ag. P, Fe, Sr and Cl which indicate that not all Ag is released from coral but still present some in phosphate compounds from SBF. Other elements of P, Fe and Sr are representing the original coral. From these results, it is clear that the samples are enriched with Ca and Ag.
 |
Fig. (7) SEM and EDX of Ag/coral (a) and (b) show massive crystallizations, cubes and needles, (c) leaf like deposition, trace of P, Fe and Sr from parent coral. |
3.2.3.b. Zn / Coral
Zn / coral sample, shows different types of crystallization which is compact and oriented parallel to the surface. Another morphology is spongy and perpendicular to surface (Fig. 8a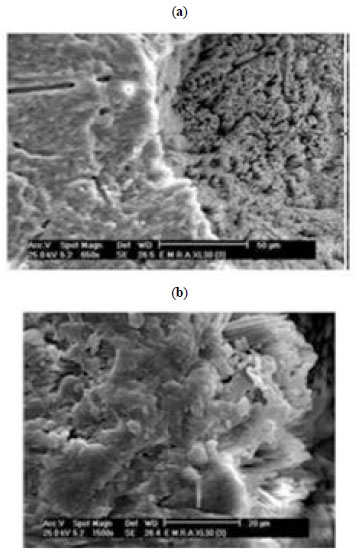 ). These parallel crystals and some plates on the septum are magnified in Fig. (8b
). These parallel crystals and some plates on the septum are magnified in Fig. (8b ).
).
 |
Fig. (8) SEM of Zn/Coral a and b. |
3.2.3.c. Sr / Coral
Sr / coral sample, (Fig. 9a ), inside the chamber shows Ca phosphate rose shapes, and the surface is full of pores denoting the dissolution of some of the formed layer by prolonged soaking in SBF. The EDX of this area revealed the presence of Ca and minor amount of Sr. The presence of Sr is originating also from parent coral having aragonite structure [12] beside impregnation with Sr 2+.
), inside the chamber shows Ca phosphate rose shapes, and the surface is full of pores denoting the dissolution of some of the formed layer by prolonged soaking in SBF. The EDX of this area revealed the presence of Ca and minor amount of Sr. The presence of Sr is originating also from parent coral having aragonite structure [12] beside impregnation with Sr 2+.
 |
Fig. (9) (a) SEM and EDX of Sr/coral a: show twined crystallization (b) show longitudinal parallel inclined needles. |
Another area with longitudinal parallel and inclined needles underneath a large mass full of rounded pores due to dissolution along with cracks is shown in Fig. (9b ). The EDX of this area reveales the presence of Ca and minor amounts of Sr.
). The EDX of this area reveales the presence of Ca and minor amounts of Sr.
During early immersion in SBF values of conductivity increase in the order of Sr2+ > Zn2+ >Ag+ coral samples and saturation levels are recorded at21, 30and 45 h respectively. Minimum values are recorded by Zn/coral while maximum ones are recorded by Sr/coral, although, the Zn/coral sample started like Sr/coral then decreased gradually to reach Ag/coral at 39 hrs of measurements. This could be related to the different sizes of the ions involved having sizes of 194, 165, 142 and 219 pm for Ca2+, Ag+, Zn2+ and Sr2+ respectively which would affect surface exchange processes.
In saline, an increase of Ca2+concentrations in an order parallel to Ag+ > Zn2+ > Sr2+ /corals are recorded. The Sr/coral records peculiar trend of minimum increase of Ca but accompanied by maximum increase in iP.
The SBF of Sr/coral records maximum value of iP which should account for its involvement in a compound forming and minimum increase of Ca. The order of highest release and concentration of Ca in SBF is parallel to of Ag, > Zn coral. The depletion of Ag, Sr or Zn ions is in favour of antibacterial and antimicrobial effects of the corals beside enhancement of the bone formation.
The values of Sr, iP and Ca2+ in the treated parent coral are 0. 7, 0. 5 and 39. 7 respectively, as measured by spectrophotometer which would account for the high released Ca 2+ in Saline and SBF solutions.
The released ions in saline are 0. 35, 0. 31and 0. 22mg for Ag, Zn and Sr respectively proving release in the same order from corresponding coral samples which would reduce its conductivity. However, conductivity values followed opposite trend. In SBF, the values of released ions are lower being 0. 30, 0. 21 and 0. 17 mg for the same order. Therefore, some of these ions are involved in the process of surface deposition of respective compounds or incorporated in phosphate phases
Silver ions have long been known to have powerful antibacterial activity [20], and the antibacterial activity is against Gram-negative Escherichia Coli (E. coli, ATCC25922) and Gram positive Staphlococcus aureus (S. aureus, ATCC6538). Striking features of Ag are reflected by clinical studies with silver based coating on medical devices. Bacteria are quite susceptible to Ag with bactericidal activity reported at Ag concentration as low as 35ppb [21].
Zinc was reported to have osteoconductive properties [22] and have direct stimulatory effect on bone mineralization in vitro and bone protein synthesis [22, 23]. Yamaguchi et al. reported an increase in protein synthesis in bone cells inducing bone formation [23].
Strontium has been reported to contribute to increase bone mass and volume when given at low doses, remineralizing skeletal lesions [24]. It seems that Sr directly suppresses bone resorption and has no deleterious effect on bone mineralization [25]. It was reported to have beneficial effects on bone formation in humans which results in increase trabecular volume [26]. The ionic strontium (Sr)in human, share the same physiologic pathway as calcium [27] and can be deposited into the mineral structure of the bone, especially in the regions of high metabolic turnover [28].
Nearly half century ago the beneficial affect of low doses of stable Sr in the treatment of osteoporosis, characterized by low bone mass, fracture risk and enhanced bone fragility was discovered [29]. The effect was attributed to preventation of bone loss by mechanical of depressing bone resorption and maintaining bone formation. According to Grynpas et al [30], the treatment with low doses of Sr increased the number of bone forming sites and vertebral bone volume in rats and showed no adverse effects on the mineral profile chemistry or bone matrix mineralization.
The conductivity values and trend are coinciding with those of biochemical data of SBF. The samples are possessing intermediate porosity of 17% with interconnectivity and architectures similar to bone. Therefore, they could be suitable for tissue engineered constructs that would possess antimicrobial effect and /or bone searching function to reduce implant infection and enhance bone formation especially with its natural architecture.
CONCLUSIONS
- The conductivity values of saline proved dissolution in a reverse trend to SBF proving ionic deposition from the latter in favor biomimetic deposition.
- As conductivity was saturated as early as 20h for Sr/corals, the immersion for such time is recommended for biolayer deposition. Saturation was recorded at 32 and 45 hrs for Ag and Zn respectively.
- The presence of Sr2+ in corals lead to higher iP release from coral which would invite bone forming process involving Ca2+ precipitation.
ACKNOWLEDGEMENTS
The authors acknowledge the support of National Research Centre and Ain Shams University for the MSc program of Hadeer Ibraheem at biophysics dept., Faculty of Science, Cairo, Egypt.
