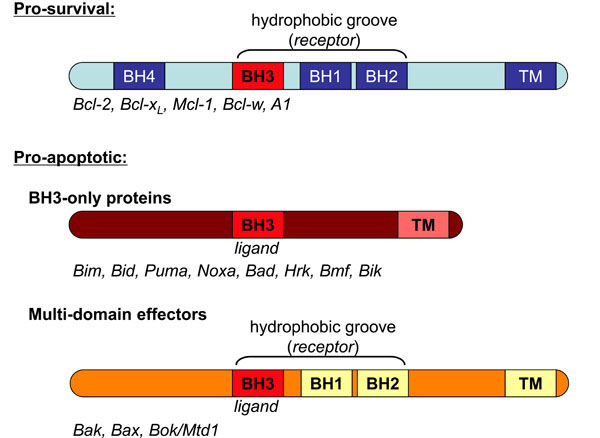
Fig. (1) Regulators of apoptosis- The Bcl-2 family. The Bcl-2 proteins form a complex network of interactions that govern susceptibility
to death stimuli. Bcl-2 homologues are characterised by regions of sequence homology, the Bcl-2 homology (BH) domains 1-4. The majority
of Bcl-2 homologues also have a hydrophobic C-terminal transmembrane (TM) domain that anchors them to intracellular membranes. Prosurvival
proteins such as Bcl-2, Bcl-xL and Mcl-1 have BH domains 1-4. Pro-survival proteins have a hydrophobic groove derived from BH
domains 1-3 that acts as a receptor surface for BH3 domain ligands from specific BH3-only proteins. The pro-apoptotic BH3-only proteins
share little sequence homology except in the BH3 domain. These proteins sense cellular stress and are activated either by transcriptional
upregulation (e.g. Noxa, Puma) or post-translational modification such as phosphorylation (e.g. Bad) or proteolytic cleavage (Bid is
converted to its truncated form, tBid). The pro-apoptotic effector proteins Bak, Bax and Bok/Mtd1 have BH1-4 and are often described as
multi-domain pro-apoptotic proteins. Bak and Bax seemingly have both the receptor and ligand attributes allowing their critical selfassociation
during cell death.