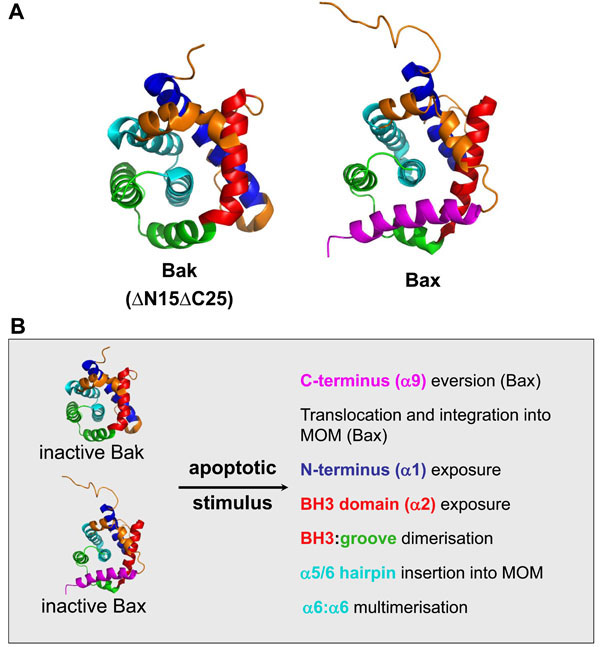
Fig. (2) Bak and Bax change conformation significantly during apoptosis. A. Inactive Bak and Bax adopt a similar tertiary structure.
Structures of Bak (ΔN15/ΔC25, 2IMS) and Bax (1F16) as determined by crystallography and nuclear magnetic resonance respectively [5,6].
Regions that undergo significant rearrangement during activation are indicated, α1 (blue), α2 (BH3, red), α5/6 (cyan) and α9 (TM,
magenta). α helices 3-4 in combination with α5 comprise the hydrophobic groove (green), which forms the docking site for self-association
and potentially for interaction with other BH3-only proteins. The Bak structure lacks both its N and C-termini. Images were generated with
MacPyMOL. B. Bak/Bax conformation change during apoptosis. Bak and Bax undergo a series of conformation changes that facilitate
subcellular redistribution (Bax), MOM integration and oligomerisation. Bax is normally cytosolic due to sequestration of its hydrophobic Cterminal
TM domain in its hydrophobic groove (green), so that its activation involves exposure of its TM domain and translocation to and
integration into the MOM. In contrast, Bak is constitutively mitochondrial via integration of its C-terminus into the MOM and therefore
bypasses this redistribution phase and ensures that its hydrophobic groove is normally unoccupied. The precise order of these events is
uncertain. The oligomerization of activated Bak involves insertion of its BH3 domain into the hydrophobic groove of a partner molecule.
These dimers then multimerise via an interface involving the α6 helices.