- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Process Chemistry Journal
(Discontinued)
ISSN: 1875-1806 ― Volume 6, 2014
Effect of Irgacure 651 Initiator on Poly(Methyl Methacrylate) Photostability Studied by UV-Vis Spectroscopy#
Halina Kaczmarek*, Piotr Gałka
Abstract
Commercial initiator – 2,2-dimethoxy-2-phenyl acetophenone (Irgacure 651, Ciba, Switzerland) was introduced to poly(methyl methacrylate) (PMMA) matrix to enhance the polymer degradation. Two types of thin polymeric films (PMMA and PMMA+5% Irgacure) were UV-irradiated in laboratory conditions (Xenon lamp, air atmosphere, room temperature). The photochemical changes in pure PMMA and PMMA doped with the initiator were studied by absorption spectroscopy in the UV-Vis range. Separately, the behaviour of the initiator in the acetonitrile (ACN) solution upon UV-radiation was investigated at the same conditions. It was found that the addition of the Irgacure 651 to PMMA accelerated the photooxidative degradation, particularly at the early stages of exposure. The efficiency of formation of chromophoric groups in PMMA in the presence of the Irgacure initiator was significantly greater than in pure polymer. This suggests that the free-radical products of the initiator photolysis (mainly benzoyl radicals) are able to abstract hydrogen atoms from PMMA molecules.
Article Information
Identifiers and Pagination:
Year: 2008Volume: 1
First Page: 8
Last Page: 11
Publisher Id: TOCPCJ-1-8
DOI: 10.2174/1875180601001010008
Article History:
Received Date: 30/7/2008Revision Received Date: 30/9/2008
Acceptance Date: 7/10/2008
Electronic publication date: 17/10/2008
Collection year: 2008
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Faculty of Chemistry, Nicolaus Copernicus University, 7, Gagarin Str., 87-100 Toruń, Poland; E-mail: halina@chem.uni.torun.pl
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 30-7-2008 |
Original Manuscript | Effect of Irgacure 651 Initiator on Poly(Methyl Methacrylate) Photostability Studied by UV-Vis Spectroscopy# | |
INTRODUCTION
Irgacure photoinitiators constitute a family of commercial compounds, produced by Ciba Specialty Chemicals, Switzerland. They are well known as efficient intiators of polymerization of various kinds of monomers [1-7]. Such initiators as benzyl ketals, α,α-dialkoxyacetophenones and α-hydroxyalkylphenones contain chromophoric groups that absorb radiation in ultraviolet or visible range and undergo photolysis with formation of free radicals. The photochemical properties and photoinitiation activity of these organic compounds in polymerization and crosslinking of acrylated monomers were studied by various experimental methods. One of the most efficient photoinitiators in this group is Irgacure 651 (DMPA), C6H5-CO-C(OCH3)2-C6H5 [1, 2]. The compound is characterized by high phosphorescence quatum yield (Φ=0.62) and relatively long triplet lifetime (τ1/2= 2.59ms). High polymerization rate and large conversion degree in the presence of DMPA is explained by the significant benzoyl radical concentration, which was confirmed by flash photolysis. The initial concentration of the initiator in photopolymerised formulation has a great influence on the molecular weight and polydispersity of the obtained polymer [8].
Although the photoinitiating action of Irgacure compounds is well documented, the idea of using of these photoinitiators as degrading agents for the purpose of obtaining of the degradable materials is rather new. Our preliminary work, considering this topic, gives the promising information useful for designing the polymeric materials with controlled life-time.
The aim of this presentation is to study the photostability of poly(methyl methacrylate) (PMMA) doped with 2,2-dimethoxy-2-phenyl acetophenone initiator (abbreviation - DMPA, trade name - ®IRGACURE 651) and to compare the course of photochemical reaction in doped and undoped polymers using UV-Vis spectroscopy.
MATERIALS AND METHODOLOGY
The poly(methyl methacrylate) (Mw =120000, Sigma-Aldrich) was used without purification. The photointiator – ®Irgacure 651 was supplied by Ciba, Basel, Switzerland. The polymer and the initiator were dissolved in tetrahydrofurane. Thin polymeric films were obtained by casting the solution directly onto spectroscopic windows. The modified PMMA contained 5%(wt) of Irgacure 651.
The polymeric samples were UV-irradiated at room temperature and in air for 1-24h using high pressure xenon lamp XBO R 180 W/45 OFR with ZXE 180 power supply adaptor (provided by Optel, Opole, Poland). The intensity of incident light, monitored by the HD 9021 radiometer (Delta, Italy), was equal to 35.2 W/cm2 (for UV-A), 9.70 W/cm2 (UV-B) and 2.30 W/cm2 (UV-C).
The UV-Vis spectra of the pure PMMA and the polymer doped with DMPA were recorded by UV-1601PC spectrometer (Shimadzu, Japan).
DMPA initiator was exposed to the radiation emitted from Xenon lamp in acetonitrile (ACN) solutions at different concentrations. The irradiation and spectroscopic studies were performed using a quartz cell with a path length of 1 cm.
RESULTS AND DISCUSSIONS
The changes of electronic spectra of Irgacure 651 in ACN solutions caused by the UV-irradiation are shown in Fig. (1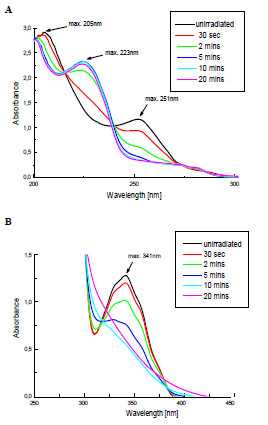 ). The main bands with the maxima at 205, 251 and 341 nm are present, which is in a good agreement with the literature data [2]. These bands are attributed to π→π* and n→π* transitions.
). The main bands with the maxima at 205, 251 and 341 nm are present, which is in a good agreement with the literature data [2]. These bands are attributed to π→π* and n→π* transitions.
 |
Fig. (1) The changes in UV-Vis absorption spectra of Irgacure 651 in ACN solutions during UV-irradiation: (A) Cm= 8.8x10-5mol/dm3; (B) Cm= 4.4x10-3mol/dm3. |
The Fig. (1A ) shows the decrease of the band intensity at 205 and 251 nm with the simultaneous formation of a new absorption peak at 223 nm during the 20 minutes of exposure. The low-intensity band at higher wavelenght (341 nm) also disapears rapidly, which is clearly observed in spectrum recorded for more concentrated solution (Fig. 1B
) shows the decrease of the band intensity at 205 and 251 nm with the simultaneous formation of a new absorption peak at 223 nm during the 20 minutes of exposure. The low-intensity band at higher wavelenght (341 nm) also disapears rapidly, which is clearly observed in spectrum recorded for more concentrated solution (Fig. 1B ). It indicates that Irgacure 651 undergoes the efficient photolysis in applied conditions.
). It indicates that Irgacure 651 undergoes the efficient photolysis in applied conditions.
The carbonyl organic compounds of benzoin type, after the photon absorption and excitation undergo the α-dissociation (Norrish type I cleavage) according to Scheme 1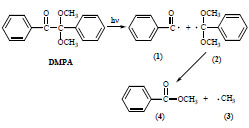 . This reaction is very fast and formed benzoyl (1) and α,α-dimethoxybenzyl radicals (2) differ significantly in their reactivity [9-13]. The fragmentation of the latter one leads to the formation of small methyl radical (3) and methyl benzoate (4). Both (1) and (3) radicals participate in photoinitiated polymerization but the action of •CH3 is much less effective than this of (1). Moreover, other major products of DMPA photolysis are benzaldehyde, benzil and acetophenone [14]. Benzil, which is diketone (C6H5C=O)2 is also used as a photoinitiator in the polymer curing.
. This reaction is very fast and formed benzoyl (1) and α,α-dimethoxybenzyl radicals (2) differ significantly in their reactivity [9-13]. The fragmentation of the latter one leads to the formation of small methyl radical (3) and methyl benzoate (4). Both (1) and (3) radicals participate in photoinitiated polymerization but the action of •CH3 is much less effective than this of (1). Moreover, other major products of DMPA photolysis are benzaldehyde, benzil and acetophenone [14]. Benzil, which is diketone (C6H5C=O)2 is also used as a photoinitiator in the polymer curing.
 |
Scheme 1 Photolysis of Irgacure 651 initiator. |
The photostability of PMMA and PMMA + Irgacure 651 films of the same thickness were investigated using UV-Vis spectroscopy. The examples of changes in UV-Vis spectra of these samples are presented in Fig. (2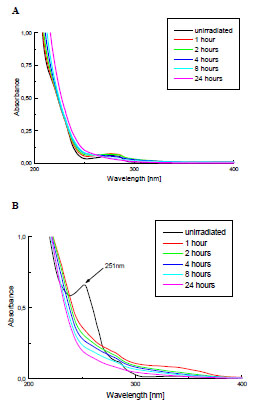 ). As can be noticed, the band at 251 nm, atributed to the photoinitiator, is present in the spectrum of the modified PMMA. However, it disapears already in the first hour of UV-irradiation.
). As can be noticed, the band at 251 nm, atributed to the photoinitiator, is present in the spectrum of the modified PMMA. However, it disapears already in the first hour of UV-irradiation.
 |
Fig. (2) Changes of UV-Vis spectra of the pure PMMA (A) and PMMA doped with Irgacure 651 (B) during the exposure to Xenon lamp. |
The other signals due to initiator are difficult to detect in PMMA spectrum. The maximum at 205 nm overlaps the carbonyl band from PMMA; the band at 341 nm, owing to its low absorption coefficient [8] and because of low DMPA concentration in polymeric sample is hardly seen.
The prolongation of exposure time causes an increase of the absorbance in the 200-400nm range. The observed changes in UV-Vis spectra of both polymeric samples are irregular - particularly fast increase of the absorbance was observed at the beginning of the irradiation. The broad absorption band formed during the sample exposure indicates that the complex mixture of photoproducts arises. The increase of the absorbance in both UV-irradiated samples can be explained by the formation of the new functional groups such as carbonyls in main chain and separated or conjugated double bonds. It is known that PMMA undergoes mainly depolymerisation but furthermore a random chain scission takes place [15]. The created monomer and oligomers containing unsaturated bonds accumulated in the matrix additionally increase the absorption of the degraded polymer.
The solvent impurities which are present in both unexposed samples have no significant effect on the photodegradation rate, what was proved in the previous experiment.
However, to investigate the kinetics of chromphore formation more thoroughly and to avoid the possible errors, the absorbance at 300 nm (which is outside of initiator absorption) was chosen for comparison the behavior of the PMMA and PMMA + DMPA samples.
Fig. (3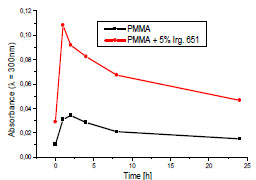 ) shows the differences in the kinetics of the photochemical reaction leading to the generation of the products absorbing at 300 nm. The rapid absorbance increase after 1h irradiation is an evidence of the fast reaction in the first period of the experiment. The next stage of photooxidative degradation is significantly restrained. The yield of the chromophoric group formation in the modified PMMA is a few times higher that in pure polymer. It is obviously caused by high concentration of the free radicals formed as a result of the DMPA decay.
) shows the differences in the kinetics of the photochemical reaction leading to the generation of the products absorbing at 300 nm. The rapid absorbance increase after 1h irradiation is an evidence of the fast reaction in the first period of the experiment. The next stage of photooxidative degradation is significantly restrained. The yield of the chromophoric group formation in the modified PMMA is a few times higher that in pure polymer. It is obviously caused by high concentration of the free radicals formed as a result of the DMPA decay.
 |
Fig. (3) The kinetics of chromophore formation in the pure PMMA and the PMMA containing 5% Irgacure 65 during the exposure to Xenon lamp. |
The enhancement of the photoreaction in the modified PMMA can be explained by the reaction of the initiating species from DMPA with macromolecules. Most probable is the hydrogen abstraction from PMMA (Scheme 2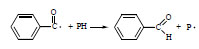 ).
).
 |
Scheme 2 Initiation of PMMA decomposition by benzoyl radical. |
The macroradical (P•) undergoes the secondary reactions, for example the addition of the oxygen molecules with the formation of peroxyradicals (POO•), which is a typical process for many polymers [15].
CONCLUSIONS
It was found that the presence of smal amount of 2,2-dimethoxy-2-phenyl acetophenone in PMMA film accelerates its photodegradation.
Photolysis of DMPA in PMMA matrix is slower than in acetonitrile solution. This is caused by trapping of the initiator in the stiff polymer matrix where the mobility of the molecules is restricted, contrary to the solution. However, the concentration of the free radicals formed during the DMPA photodecomposition is high enough for the initiation of the macromolecules scission. The photooxidative degradation, leading to the chromophore formation, occurs in the doped PMMA with the higher rate and efficiency comparing to the virgin PMMA.
NOTES
# Presented on the 42nd IUPAC World Polymer Congress, Polymers at Frontiers of Science and Technology, Taiwan, Taipei, 2008.
ACKNOWLEDGEMENTS
The financial support from a Rector grant (Nicolaus Copernicus University, Toruń, Poland, grant number: 561-Ch) is gratefully acknowledged.
