- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Food Science Journal
(Discontinued)
ISSN: 1874-2564 ― Volume 13, 2021
Antioxidant Activity and Bioactive Compounds of Babassu (Orbignya phalerata) Virgin Oil Obtained by Different Methods of Extraction
Luciana Carolina Bauer1, Ellen Cristina Quirino Lacerda2, Leandro Soares Santos1, Sibelli Passini Barbosa Ferrão1, Rafael da Costa Ilhéu Fontan1, Cristiane Martins Veloso1, Renata Cristina Ferreira Bonomo1, *
Abstract
Background:
The investigation of new sources of raw materials and the knowledge of the composition of the food is fundamental for the evaluation of their potential and the availability of nutrients for the consumer population.
Objective:
This work aimed to deepen the knowledge about the crude oil of babassu fruit obtained by two different methods of extraction, cold pressing and extraction by cooking the fruit almond.
Method:
Total phenolic compounds contents and antioxidant activity were determined by ferric reducing antioxidant potential assay and 2,2-diphenyl-1-picrylhydrazyl radical scavenging capacity assay. By liquid chromatography, the content of different bioactive compounds was determined. Data was submitted to Analysis of Variance (ANOVA) and compared by f test (p <0.05).
Results:
The results showed that for most of the bioactive compounds there was no difference between the two types of babassu oil. For those compounds where the oils differed, the virgin oil had about three times the content of the extra-virgin oil. In addition, the antioxidant activity was higher for the oil extracted by cooking of the babassu mass, ranging from approximately 2.5 times higher up to 19.2 times higher than the antioxidant activity of the babassu oil extracted by pressing.
Conclusion:
The process of extraction by cooking the almond mass can incorporate a larger number of bioactive components and improve the antioxidant activity of the virgin babassu oil. However, the extraction method does not influence the content of tocopherols of distinct types of babassu oil.
Article Information
Identifiers and Pagination:
Year: 2019Volume: 11
First Page: 35
Last Page: 43
Publisher Id: TOFSJ-11-35
DOI: 10.2174/1874256401911010035
Article History:
Received Date: 29/9/2018Revision Received Date: 4/12/2018
Acceptance Date: 30/12/2018
Electronic publication date: 28/03/2019
Collection year: 2019
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Laboratory of Process Engineering, State University of Bahia (UESB), Campus Juvino Oliveira, Praça Primavera, 40, Itapetinga, Bahia, Brazil; Tel: +55-77-3261-8659; E-mail: bonomorcf@yahoo.com.br
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 29-9-2018 |
Original Manuscript | Antioxidant Activity and Bioactive Compounds of Babassu (Orbignya phalerata) Virgin Oil Obtained by Different Methods of Extraction | |
1. INTRODUCTION
Vegetable oils are those obtained from parts of plants, such as seeds, nuts, flowers and fruit pulp, and vegetables and legumes mainly formed by lipids. They are the most produced and consumed oils in the world [1 USDA - United States Department of Agriculture. Oil Seeds: World Markets and Trad/ September. [updated 2018 Set 22; cited 2018 Set 22] (2018). Available from:
https:/ /www.fas. usda. gov/ data/ oilseeds-world-markets-and-trade.] and represent significant importance in the population’s diet. They are sources of saturated and unsaturated fatty acids, liposoluble vitamins and antioxidants [2Kumar A, Sharma A, Upadhyaya KC. Vegetable oil: Nutritional and industrial perspective. Curr Genomics 2016; 17(3): 230-40.
[http://dx.doi.org/10.2174/1389202917666160202220107] [PMID: 27252590] , 3Savva SC, Kafatos A. Vegetable oils: Dietary importance. Encyc-
lopedia of Food and Health 2016; 365-72.].
Vegetable oils are versatile raw materials for the food, pharmaceutical and chemical industries, as they have different compositions and technological uses [3Savva SC, Kafatos A. Vegetable oils: Dietary importance. Encyc-
lopedia of Food and Health 2016; 365-72., 4Hettiarachchi D, Liu Y, Fox B, Sunderland B. Westem Australian sandal wood seed oil: New opportunities. Lipid Technol 2010; 22(2): 27-9.
[http://dx.doi.org/10.1002/lite.200900071] ]. For example, oils from oilseeds are rich in polyunsaturated fatty acids and vitamins [3Savva SC, Kafatos A. Vegetable oils: Dietary importance. Encyc-
lopedia of Food and Health 2016; 365-72., 5Ergönül PG, Köseoğlu O. Changes in α, β, γ, δ-tocopherol contents of mostly consumed vegetable oils during refining process. CYTA J Food 2014; 12(2): 199-02.
[http://dx.doi.org/10.1080/19476337.2013.821672] , 6Kerrihard AL, Pegg RB. Utilizing the bioactive contents of specialty oils and fats. Specialty Oils and Fats in Food and Nutrition - Properties, Processing and Applications 2015; 317-48.
[http://dx.doi.org/10.1016/B978-1-78242-376-8.00013-2] ], fruit pulp oils such as oil palm and olive oil have a high amount of antioxidant compounds [7Ghazani SM, Marangoni AG. Healthy fats and oils 2016.-9Del Monaco G, Officioso A, D’Angelo S, et al. Characterization of extra virgin olive oils produced with typical Italian varieties by their phenolic profile. Food Chem 2015; 184: 220-8.
[http://dx.doi.org/10.1016/j.foodchem.2015.03.071] [PMID: 25872 448] ] and palm kernel oil has a large amount of saturated fatty acids [10Oliveira RAD, Neves SC, Ribeiro LM, Lopes PSN, Silvério FO. Storage, oil quality and cryopreservation of babassu palm seeds. Ind Crops Prod 2016; 91: 332-9.
[http://dx.doi.org/10.1016/j.indcrop.2016.07.039] , 11Bauer LC, Damásio JMA, Da Silva MV, Santana DA, Gualberto AS, Simionatto JI. Chemical characterization of pressed and refined licuri (Syagrus coronata) oils. Acta Sci Technol 2013; 35(4): 771-6.
[http://dx.doi.org/10.4025/actascitechnol.v35i4.20251] ]. These different compositions allow vegetable oils to be applied to various products. In the food industry, they are used to cook or fry [12Waghmare A, Patil S, LeBlanc JG, Sonawane S, Ayra SS. Comparative assessment of algal oil with other vegetable oils for deep frying. Algal Res 2018; 31: 99-106.
[http://dx.doi.org/10.1016/j.algal.2018.01.019] ,13Meinhart AD, da Silveira TFF, de Moraes MR, et al. Optimization of frying oil composition rich in essential fatty acids by mixture design. LWT 2017; 84: 795-803.
[http://dx.doi.org/10.1016/j.lwt.2017.06.053] ], as ingredients of different food products, creams and margarine [14Guillén MD, Ibargoitia ML, Sopelana P. Margarine: Composition and analysis 2016; 646-53.-16Lopes CO, Barcelos MFP, Dias NAA, Carneiro JDS, De Abreu WC. Effect of the addition of spices on reducing the sodium content and increasing the antioxidant activity of margarine. LWT 2014; 58: 63-70.
[http://dx.doi.org/10.1016/j.lwt.2014.02.029] ], bakery products [17Talbot G. Specialty oils and fats in confectionery. Specialty Oils and Fats in Food and Nutrition 2015; 221-39.
[http://dx.doi.org/10.1016/B978-1-78242-376-8.00009-0] ], ice cream [18Smith KW. Specialty oils and fats in ice cream. Specialty Oils and Fats in Food and Nutrition 2015; 271-84.
[http://dx.doi.org/10.1016/B978-1-78242-376-8.00011-9] ], and chocolates [19Halim HSA, Selamat J, Mirhosseini SH, Hussain N. Sensory preference and bloom stability of chocolate containing cocoa butter substitute from coconut oil. J Saudi Soc Agric Sci 2018.
[http://dx.doi.org/10.1016/j.jssas.2018.02.005] ]. In the pharmaceutical industry these oils are used in cosmetics [20Pinto F, De Barros DPC, Fonseca LP. Design of multifunctional nanostructured lipid carriers enriched with α-tocopherol using vegetable oils. Ind Crops Prod 2018; 118: 149-59.
[http://dx.doi.org/10.1016/j.indcrop.2018.03.042] , 21Lacatusu I, Arsenie LV, Badea G, Popa O, Oprea O, Badea N. New cosmetic formulations with broad photoprotective and antioxidative activities designed by amaranth and pumpkin seed oils nanocarriers. Ind Crops Prod 2018; 123: 424-33.
[http://dx.doi.org/10.1016/j.indcrop.2018.06.083] ] and therapeutic formulations [22Beidokhti MN, Jäger AK. Review of antidiabetic fruits, vegetables, beverages, oils and spices commonly consumed in the diet. J Ethnopharmacol 2017; 201: 26-41.
[http://dx.doi.org/10.1016/j.jep.2017.02.031] [PMID: 28257977] , 23Al-Attar AM, Elnaggar MHR, Almalki EA. Protective effect of some plant oils on diazinon induced hepatorenal toxicity in male rats. Saudi J Biol Sci 2017; 24(6): 1162-71.
[http://dx.doi.org/10.1016/j.sjbs.2016.10.013] [PMID: 28855808] ].
Thus, the world demand for oils and fats is increasing, so several researchers are engaged in finding new sources of vegetable oils with high nutritional quality, industrial importance and pharmaceutical activity [24Feranil AB, Duazo PL, Kuzawa CW, Adair LS. Coconut oil is associated with a beneficial lipid profile in pre-menopausal women in the Philippines. Asia Pac J Clin Nutr 2011; 20(2): 190-5.
[PMID: 21669587] -31Ganesan K, Sukalingam K, Xu B. Impact of consumption and cooking manners of vegetable oils on cardiovascular diseases A critical review. Trends Food Sci Technol 2018; 71: 132-54.
[http://dx.doi.org/10.1016/j.tifs.2017.11.003] ]. Brazil has great biodiversity and therefore an infinity of plants potentially producing oil.
Babassu (Orbignya phalerata) is a palm commonly found in the North and Northeast regions of Brazil [32Teixeira MA. Babassu–A new approach for an ancient Brazilian biomass. Biomass Bioenergy 2008; 32: 857-64.
[http://dx.doi.org/10.1016/j.biombioe.2007.12.016] ]. Babassu is a valuable resource in these regions both in economic and nutritional terms [33IBGE – Instituto Brasileiro de Geografia e Estatística. Produção da Extração Vegetal e da Silvicultura 2015; 30: 1-48.]. This importance is related to the exploitation of the culture [34Carrazza LR, Ávila JCC, Silva ML. Manual Tecnológico de Aproveitamento Integral do Fruto e da Folha do Babaçu (Attalea spp) 2nd ed. 2012; 68.], which is extractive and exploited by low-income people, as well as the substantial number of products and by-products that can be originated using this fruit. Babassu oil is the main product, usually used in the diet of the population of these regions [10Oliveira RAD, Neves SC, Ribeiro LM, Lopes PSN, Silvério FO. Storage, oil quality and cryopreservation of babassu palm seeds. Ind Crops Prod 2016; 91: 332-9.
[http://dx.doi.org/10.1016/j.indcrop.2016.07.039] , 32Teixeira MA. Babassu–A new approach for an ancient Brazilian biomass. Biomass Bioenergy 2008; 32: 857-64.
[http://dx.doi.org/10.1016/j.biombioe.2007.12.016] , 34Carrazza LR, Ávila JCC, Silva ML. Manual Tecnológico de Aproveitamento Integral do Fruto e da Folha do Babaçu (Attalea spp) 2nd ed. 2012; 68.] or for the production of cosmetics and hygiene and cleaning products [35Soler MP, Vitali AA, Muto EF. Tecnologia de quebra do coco babaçu (Orbignya speciosa). Food Sci Technol (Campinas) 2007; 27(4): 717-22.
[http://dx.doi.org/10.1590/S0101-20612007000400007] , 36Teixeira MA. Heat and power demands in babassu palm oil extraction industry in Brazil. Energy Convers Manage 2005; 46: 2068-74.
[http://dx.doi.org/10.1016/j.enconman.2004.10.014] ], and, also for the production of biofuels [36Teixeira MA. Heat and power demands in babassu palm oil extraction industry in Brazil. Energy Convers Manage 2005; 46: 2068-74.
[http://dx.doi.org/10.1016/j.enconman.2004.10.014] -38Paiva EJM, da Silva MLCP, Barboza JCS, de Oliveira PC, de Castro HF, Giordani DS. Non-edible babassu oil as a new source for energy production A feasibility transesterification survey assisted by ultrasound. Ultrason Sonochem 2013; 20(3): 833-8.
[http://dx.doi.org/10.1016/j.ultsonch.2012.11.003] [PMID: 23207058] ], being the by-products destined for other uses, such as the use of the defatted cake for animal feed [34Carrazza LR, Ávila JCC, Silva ML. Manual Tecnológico de Aproveitamento Integral do Fruto e da Folha do Babaçu (Attalea spp) 2nd ed. 2012; 68., 39Fonseca FLR, Siqueira JC, Vaz RGMV, et al. Os Benefícios do Babaçu na Alimentação das Aves – Revisão de Literatura 2014; 23., 40Albuquerque NI, Contreras CC, Alencar S, et al. Propriedades da carne e perfil de ácidos graxos do pernil de catetos (Tayassu tajacu) alimentados com torta de babaçu (Orbignya phalerata). Arq Bras Med Vet Zootec 2009; 61(6): 1419-27.
[http://dx.doi.org/10.1590/S0102-09352009000600023] ].
According to Carazza et al. (2012), there are basically two forms of processing to obtain babassu oil, both handmade in domestic or cooperative form. One process is performed by cold mechanical pressing and, in the other process, by cooking the mass of babassu crushed almonds. Extra-virgin babassu oil is obtained by cold pressing whole, healthy almonds without yellowing or superficial physical damage, followed by decantation, filtration and packaging. The virgin babassu oil is obtained by crushing previously selected and toasted almonds, followed by baking the mass to obtain the oil, which is separated by decanting, filtered and reheated for total removal of the water resulting from the cooking step and then packaged.
Babassu oil is composed mainly of saturated fatty acids (80-91%), such as lauric acid (43-50%), myristic acid (15- 18%), palmitic acid (6-10%), capric acid (4-6%), caprylic acid (0-5%) and stearic acid (3-5%); the remainder is unsaturated fatty acids (9-20%), in which oleic acid (12-19%) and linoleic acid (1-3%) are present [10Oliveira RAD, Neves SC, Ribeiro LM, Lopes PSN, Silvério FO. Storage, oil quality and cryopreservation of babassu palm seeds. Ind Crops Prod 2016; 91: 332-9.
[http://dx.doi.org/10.1016/j.indcrop.2016.07.039] , 38Paiva EJM, da Silva MLCP, Barboza JCS, de Oliveira PC, de Castro HF, Giordani DS. Non-edible babassu oil as a new source for energy production A feasibility transesterification survey assisted by ultrasound. Ultrason Sonochem 2013; 20(3): 833-8.
[http://dx.doi.org/10.1016/j.ultsonch.2012.11.003] [PMID: 23207058] , 41Machado GC, Chaves JB, Antoniassi R. Composição em Ácidos Graxos e Caracterização Física e Química de óleos de babaçu. Rev Ceres 2006; 53(308): 463-70.]. This composition is like that of palm and coconut oil and has already been reported in the literature [42Medeiros VF, Azevedo ÍM, Carvalho MD, Egito ES, Medeiros AC. Effects of cococonut water and simvastatin in the treatment of sepsis and hemorrhagic shock in rats. Acta Cir Bras 2016; 31(12): 826-33.
[http://dx.doi.org/10.1590/s0102-865020160120000008] [PMID: 28076507] -44Satchithanandam S, Reicks M, Calvert RJ, Cassidy MM, Kritchevsky D. Coconut oil and sesame oil affect lymphatic absorption of cholesterol and fatty acids in rats. J Nutr 1993; 123(11): 1852-8.
[http://dx.doi.org/10.1093/jn/123.11.1852] [PMID: 8229300] ].
Currently, babassu oil is less used in the food industry, its major use is in the personal care industry, in the manufacture of surfactants, soaps and cosmetics in general. This is mainly because babassu oil has a high capacity to form soaps and possesses high emollient power [45Zocchi G. Skin-Feel Agents.Paye AOBM, Maibach HI (Eds), Handbook of Cosmetic Science and Technology, Marcel-Dekker Inc, New York 2001; 357-71.]. To obtain information that may stimulate other applications of this native Brazilian raw material, the objective of this study was to investigate the content of bioactive compounds and the antioxidant activity of different types of crude babassu oil obtained employing the two most used forms of extraction.
2. MATERIAL AND METHODS
2.1. Samples
The samples of babassu oil were obtained directly from producers in the states of Tocantins and Maranhão, Brazil. Extra-Virgin Babassu Oil (EVBO) was obtained by cold pressing whole, healthy almonds without yellowing or superficial physical damage, followed by decantation, filtration and packaging. The Virgin Babassu Oil (VBO) was obtained by crushing previously selected and toasted almonds, followed by baking the mass to obtain the oil, which was separated by decanting, filtered and reheated for total removal of the water resulting from the cooking step and then packaged.
2.2. Chemical Reagents
During the experiments, the following solvents and reagents were used: methanol with purity > 99% (Ecibra, Santo Amaro, São Paulo, Brazil) hexane with purity 98.5% (Synth, Diadema, São Paulo, Brazil), ferric chloride hexahydrate with purity 97% (Sigma-Aldrich, Sant Louis, USA) and ferrous sulfate heptahydrate with purity ≥ 99% (Sigma-Aldrich, Sant Louis, USA). Acetonitrile, methanol, hexane, isopropanol and acetic acid (Sigma-Aldrich, Sant Louis, USA) (both of whit purity ≥ 99.9%). Deionized water (deionizer Marte Científica, Santa Rita do Sapucaí, Minas Gerais, Brazil) and ultrapure water (Ultra water purifier MS2000, Gehaka, São Paulo, Bazil).
Folin-Ciocalteau reagents, DPPH radical solution (2,2_-diphenyl-1-picrylhydrazyl), TPTZ (2,4,6-Tris (2-pyridyl) -s-triazine), all from Sigma-Aldrich (Sant Louis, USA) were used for the analysis of antioxidant activity.
The analytical standards for the chromatographic analyzes were: gallic whit purity ≥ 99.9%, caffeic acid whit purity ≥ 98%, catechin whit purity ≥ 99%, quercetin with purity ≥ 99%, rutin with purity ≥ 96%, α-tocopherol with purity ≥ 96%, β-tocopherol, γ-tocopherol with purity ≥ 96% and δ-tocopherol with purity ≥ 96%, lycopene with purity ≥ 95%, β-carotene with purity ≥ 97% and α-carotene with purity ≥ 95%, all from Sigma-Aldrich (Sant Louis, USA).
2.3. Methods
2.3.1. Antioxidant Activity and Bioactive Compound Content Assays
The analyses of antioxidant capacity and phenolic compound content of crude babassu oils (EVBO and VBO) were carried out from the methanolic extracts, as explained below, of the samples. The quantification of tocopherols and carotenoids was performed directly for the samples of the oils.
2.3.1.1. Obtention of the Methanolic Extracts of Crude Babassu Oil Samples
The extracts were obtained according to the procedure described by Montedoro et al. [46Montedoro G, Servilli M, Baldioli M, Miniati E. Simple and hydrolyzable phenolic compounds in virgin olive oil. 1. Their extraction, separation, and quantitative and semiquantitative evaluation by HPLC. J Agric Food Chem 1992; 40: 1571-6.
[http://dx.doi.org/10.1021/jf00021a019] ]. Approximately 5.0 g of each type of oil was mixed with 1.0 mL of methanol/water solution (80:20 v/v) and vortexed for 2 min. This mixture was centrifuged at 1080g for 10 min and the methanolic portion was collected. These steps were repeated 3 more times and the supernatants were combined to form the extract.
2.3.1.2. Antioxidant Activity Assays
The total phenolic compounds of the samples [47Paradiso VM, Clemente A, Summo C, Pasqualone A, Caponio F. Extraction of phenolic compounds from extra virgin olive oil by a natural deep eutectic solvent: Data on UV absorption of the extracts. Data Brief 2016; 8: 553-6.
[http://dx.doi.org/10.1016/j.dib.2016.05.076] [PMID: 27504478] -48Franco MN, Galeano-Díaz T, López O, et al. Phenolic compounds and antioxidant capacity of virgin olive oil. Food Chem 2014; 163: 289-98.
[http://dx.doi.org/10.1016/j.foodchem.2014.04.091] [PMID: 24912 728] ] were determined using the Folin-Ciocalteau Reagent (FCR) and solutions of gallic acid with different concentrations for the construction of the analytical curve, ranging from 0.01 to 0.1 mg/mL. 0.2 mL aliquots of the methanolic extracts were mixed at 0.2 mL of FCR. After 4 min, 1.6 mL of 5% (m/v) calcium carbonate aqueous solution was added. The mixture remained 20 min in thermostated bath at 40°C and then the assays were monitored on a Shimadzu UV-1800 spectrophotometer (Shimadzu, Kyoto, Japan). The absorbance was measured at 750 nm against a blank which was methanol. The total content of phenolic compounds in the extracts was expressed in mg of gallic acid equivalent per 100 g of babassu oil.
The antiradical activity of the oils was measured using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical following the procedure described by Franco et al. (2014). Aliquots of 0.1 mL of methanolic solution of the babassu oil extracts (4 different concentrations) were mixed with 3.9 mL of the 60 μM DPPH solution. These mixtures were held in the dark for 30 min and then the absorbance was measured at 515 nm against a blank which was methanol. The percentage of inhibition of the radical was calculated according to Equation 1 and these results were used to obtain a curve relating the percentage of inhibition of the radical versus the concentration of the extract. Through linear regression, the IC50 was calculated which is the value that estimates the antioxidant concentration required to inhibit 50% of the DPPH radical.
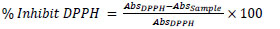 |
(1) |
Where:
AbsDPPH is the measured absorbance for the solution containing only the radical DPPH (UA);
AbsSample is the absorbance measured after the reaction between the extracts of the samples and the radical DPPH (UA).
Antioxidant activity using the ferric reducing method (FRAP) was evaluated as described by Benzie & Strain [49Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem 1996; 239(1): 70-6.
[http://dx.doi.org/10.1006/abio.1996.0292] [PMID: 8660627] ], with some modifications. Aliquots of 0.9 mL of the methanolic extracts of babassu oils, plus 0.27 mL of distilled water and 2.7 mL of the FRAP reagent were homogenized and incubated at 37° C for 30 minutes. After this period a reading at 595 nm was carried. To obtain the analytical curve, the same procedure was repeated by replacing the aliquots of the extract by ferrous sulfate solutions with different concentrations ranging from 0.15 mM to 5 mM. Thus, the results were expressed in mmol of ferrous sulphate/g of babassu oil.
2.3.1.3. Contents of Bioactive Compounds
2.3.1.3.1. Phenolic Compounds
The presence of different phenolic acids and flavonoids, namely gallic acid, caffeic acid, catechin, quercitin and rutin, were investigated. For the identification and quantification of these bioactive compounds, the methanolic extracts of the EVBO and VBO samples were obtained as described previously in item 2.3.1.1. These extracts were purified by the procedure also described by Montedoro et al. [46Montedoro G, Servilli M, Baldioli M, Miniati E. Simple and hydrolyzable phenolic compounds in virgin olive oil. 1. Their extraction, separation, and quantitative and semiquantitative evaluation by HPLC. J Agric Food Chem 1992; 40: 1571-6.
[http://dx.doi.org/10.1021/jf00021a019] ]. The methanolic extract was rotoevaporated at 30° C until complete evaporation of the solvent, and then resuspended in 1.0 mL of acetonitrile. This solution was washed three times with 1.0 mL of hexane and the acetonitrile layer was separated and evaporated at 30° C. The resulting residue was dissolved in 1.0 mL of chromatographic grade methanol and filtered through a 0.45 μm pore syringe filter.
The analyzes were performed using a liquid chromatograph (Shimadzu, Kyoto, Japan), equipped with a quaternary pump system, degasser, injection valve with 20 μL sampling loop, column furnace and diode arrangement detector. The phenolic compounds were separated on C18 reverse phase analytical column (0.25 m x 4.6 mm d.i. x 5μm particle size) (Supelco Analytical, Bellefonte, USA).
The operating parameters of the chromatograph were established as described by Azevedo et al. [50Azevedo RSA, Teixeira BS, Sauthier MCS, Santana MVA, Santos WNL, Santana DA. Multivariate analysis of the composition of bioactive in tea of the species Camellia sinensis. Food Chem 2018.
[http://dx.doi.org/10.1016/j.foodchem.2018.04.030] [PMID: 3029 2372] ]. The mobile phase consisted of a mixture of two solvents, acidified water (98: 2 v/v acetic acid water) (Solvent A) and methanol (Solvent B). Elution occurred in a gradient ranging from 100% A to 50% A and 50% B in 5 min. Passing to 35% A and 65% B in 7 min and remaining in this proportion up to 10 min. Between 10 and 12 min returning the condition 50% A and 50% B and passing to 100% A in 15 min, thus remaining up to 18 min for column stabilization and preparation for a new run. The mobile phase flow was adjusted to 1.0 mL/min and the column furnace temperature remained at 40 ° C throughout the run.
Identification of the phenolic compounds was performed by comparing the peak retention time of the samples with the peak retention time of gallic acid, caffeic acid, catechin, quercetin and rutin and by the characteristic wavelength of each substance. Chromatograms were processed at 280 nm for gallic acid and catechin, 330 nm for caffeic acid and 360 nm for quercitin and rutin [50Azevedo RSA, Teixeira BS, Sauthier MCS, Santana MVA, Santos WNL, Santana DA. Multivariate analysis of the composition of bioactive in tea of the species Camellia sinensis. Food Chem 2018.
[http://dx.doi.org/10.1016/j.foodchem.2018.04.030] [PMID: 3029 2372] ].
The quantification of the phenolic compounds was done through external standardization. The analytical curves were constructed by injecting solutions of the standards with concentrations ranging from 0.2 x 10-3 to 0.2 μg/mL and the phenolic amounts present in the EVBO and VBO were calculated using the equations of the lines.
2.3.1.3.2. Tocopherols and Carotenoids
The presence of non-esterified tocopherols and carotenoids was investigated. For identification and quantification of these compounds, 0.05 g of each of the oils were weighed and 950 μl of HPLC grade hexane was added. The mixture was run on a vortex type stirrer (Labnet International Inc., Edison, New Jersey, EUA) for 30 seconds and centrifuged in the microcentrifuge MiniStar (VWR Colletion, Vienna, Austria) at 1080g for 5 min. The supernatant was collected and filtered through a 0.45 μm pore diameter syringe filter [51Flakelar CL, Prenzler PD, Luckett DJ, Howitt JÁ, Doran G. A rapid method for the simultaneous quantification of the major tocopherols, carotenoids, free and esterified sterols in canola (Brassica napus) oil using normal phase liquid chromatography. Food Chem 2017; 214: 147-55.
[http://dx.doi.org/10.1016/j.foodchem.2016.07.059] [PMID: 2750 7459] ].
The analyzes were performed using a Shimadzu liquid chromatograph (Kyoto, Japan), equipped with a quaternary pump system, degasser, injection valve with 20 μL sampling loop, column furnace and diode arrangement detectors and fluorescence. The analytes were separated on Zorbax-SIL normal phase analytical column (0.25 m x 4.6 mm d.i. x 5μm particle size) (Supelco Analytical, Bellefonte, USA).
The mobile phase consisted of a mixture of hexane: isopropanol (99:1, v/v), elution being isocratic. The mobile phase flow was adjusted to 1.0 mL/min and the column furnace temperature remained at 25 ° C throughout the run [52Pyka A, Sliwiok J. Chromatographic separation of tocopherols. J Chromatogr A 2001; 935(1-2): 71-6.
[http://dx.doi.org/10.1016/S0021-9673(01)00944-X] [PMID: 11762 786] ].
The identification of the compounds was performed by comparing the sample retention time with the peak retention time of the carotenoid standards (lycopene, β-carotene and α-carotene) and tocopherols (α, β, γ and δ- tocopherols) and also by the characteristic wavelength of each substance. Chromatograms were processed at 290 nm (excitation) and 330 nm (emission) at the fluorescence detector for the tocopherols; and, between 390 and 700 nm, in scan mode, in the diode arrangement detector for carotenoids [53Rahmani M, Csallany AS. Chlorophyll and B-carotene pigments in Moroccan virgin olive oils measured by HPLC. J Am Oil Chem Soc 1991; 68: 672-4.
[http://dx.doi.org/10.1007/BF02662293] , 54Tan B, Brzuskiewicz L. Separation of tocopherol and tocotrienol isomers using normal and reverse-phase liquid chromatography. Anal Biochem 1989; 180(2): 368-73.
[http://dx.doi.org/10.1016/0003-2697(89)90447-8] [PMID: 2817368] ].
The quantification of tocopherols was done through external standardization. The analytical curves were constructed by injecting solutions of the standards with concentrations ranging from 0.01 to 1.5 μg/mL. The amounts of these compounds present in EVBO and VBO were calculated using the equations of the lines obtained for each curve. Curves were not constructed for carotenoids because they were not detected in the samples.
2.3.2. Statistical Analysis
The data obtained in this study, both for the antioxidant activity and for the content of bioactive compounds, was submitted to analysis of variance (ANOVA) using the SAS program, Studio version.
The antioxidant activity and contents of the different phenolic compounds and tocopherols were expressed as mean ± standard deviation and were compared using the f test (p <0.05).
3. RESULTS
3.1. Antioxidant Activity
The determination of the antioxidant capacity is based mainly on two mechanisms of reaction, the transfer of a hydrogen atom and / or the transfer of an electron. In addition to the mechanism, the objective is to determine the protective effect of the material against free radicals, which differ in the initiator radical, reaction kinetics and side reactions [55Apak R, Özyürek M, Güçlü K, Çapanoğlu E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and Electron Transfer (ET)- Based assays. J Agric Food Chem 2016; 64(5): 997-1027.
[http://dx.doi.org/10.1021/acs.jafc.5b04739] [PMID: 26728425] ]. Thus in investigating the total antioxidant capacity of a substance, it is important that at least one test of each mechanism is used. Thus, the samples were tested for the DPPH radioactivity and ferric reducing antioxidant potential assay (FRAP). In the DPPH assay both mechanisms are involved and in the FRAP assay the transfer of a hydrogen atom is involved [56Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K. Methods for testing antioxidant activity. Analyst (Lond) 2002; 127(1): 183-98.
[http://dx.doi.org/10.1039/b009171p] [PMID: 11827390] -58Alves CQ, David JM, David JP, Bahia MV, Aguiar RM. Métodos para determinação de atividade antioxidante in vitro em substratos orgânicos. Quim Nova 2010; 33(10): 2202-10.
[http://dx.doi.org/10.1590/S0100-40422010001000033] ].
The results for the determinations of the antioxidant activity of the different babassu oils studied (EVBO and VBO) can be observed in Table 1. Regardless of the test used, the antioxidant activity was higher for the oil extracted by cooking the babassu mass, ranging from ≈ 9.3 times higher up to 19.7 times higher than for the antioxidant activity of the extra virgin babassu oil.
Results presented as means ± standard deviations of the analyzes of each type of babassu oil. Results followed by different letters in the same line are significantly different by the f test at 5% probability.
3.2. Contents of Bioactive Compounds
Chromatographic conditions were suitable for separating the different phenolic compounds, gallic acid, caffeic acid, catechin, quercetin and rutin (Fig. 1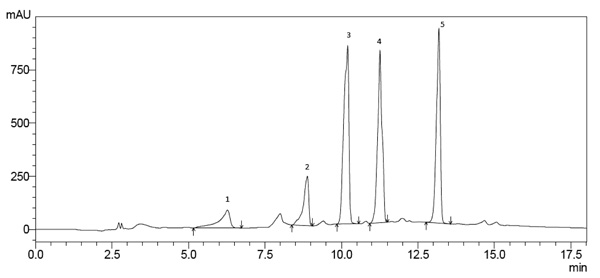 ) and tocopherols (α, β, γ, and δ-tocopherol) (Fig. 2
) and tocopherols (α, β, γ, and δ-tocopherol) (Fig. 2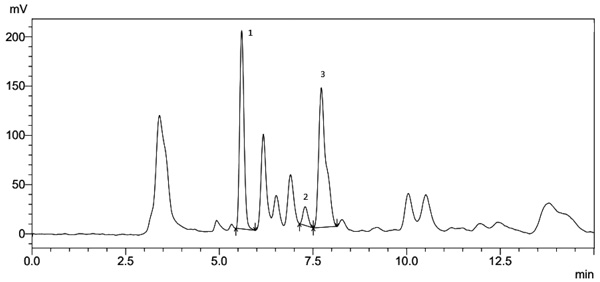 ) present in the babassu oil samples.
) present in the babassu oil samples.
The results for the quantification of the bioactive compounds, phenolics, tocopherols and carotenoids in the different babassu oils (EVBO and VBO) can be observed in Table 2. For most of the compounds, there was no difference between the types of oil. In cases where the oils differed, VBO presented content about three times higher than EVBO, except for gallic acid, where this ratio was reversed. With regard to catechin, it can be observed that for the EVBO samples the presented values are low and with high variability. Thus, the presence of this compound can be confirmed, but it is not possible to determine in what quantities.
Results presented as means ± standard deviations of the analyzes of each type of babassu oil. Results followed by different letters in the same line are significantly different by the test f at 5% probability.
 |
Fig. (1) Chromatogram characteristic of babassu oil samples. Separation of different phenolic compounds: 1) Gallic acid; 2) Catechin; 3) Caffeic acid; 4) Routine; 5) Quercitina. |
 |
Fig. (2) Chromatogram characteristic of babassu oil samples. Separation of the different tocopherols: 1) α-tocopherol, 2) β-tocopherol and 3) γ-tocopherol. |
4. DISCUSSION
4.1. Antioxidant Activity
Analyzes for concentrations of total phenolic compounds showed a significant difference between the types of babassu oil studied. Phenolic compounds are substances that have structures with aromatic rings and double conjugated bonds from which they exert their antioxidant action, besides being the most abundant antioxidants in the diet [59Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects – A review. J Funct Foods 2015; 18: 820-97.
[http://dx.doi.org/10.1016/j.jff.2015.06.018] ]. They are generally determined via reaction with the Folin-Ciocalteau reagent. However, this reagent is not only sensitive to phenolic compounds [60Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 2005; 53(10): 4290-302.
[http://dx.doi.org/10.1021/jf0502698] [PMID: 15884874] ]. Other structures with reducing power may also influence the results, for example of conjugated aldehydes, ketones, dienes and trienes, in addition to the melanoidins.
The process of obtaining VBO has two important stages of heating. At first, the mass of crushed kernel is cooked with a little water for oil scoring, which by being less dense floats on the cake and can be separated. In the second stage, this oil collected, still has a certain amount of water and therefore goes through an evaporation process. Thus, this exposure to heat may have favored oxidation and darkening reactions and consequently raised the total phenolic values of virgin babassu oil.
The oxidation of fatty acids leads to the formation of compounds such as aldehydes, ketones, dienes and conjugated trienes, among others [61McClemenets DJ, Decker EA. Lipids Fenema’s food chemistry 2007; 155-216.]. And melanoidins are heterogeneous brown pigments that have aromatic rings, are formed by reactions of non-enzymatic browning [62Mesias M, Delgado-Andrade C. Melanoidins as potential functional food ingradient. Curr Opin Food Sci 2017; 14: 37-42.
[http://dx.doi.org/10.1016/j.cofs.2017.01.007] , 63D. J., & Huber, E. A.Carbohydrates Fenema’s food chemistry 2007; 75-130.]. These pigments are produced during the Maillard reaction. This reaction occurs between the carbonyl groups of reducing sugars and the amino groups of amino acids, peptides or proteins, and is catalyzed by heating [64Ramonaityte DT, Kersiene M, Adams A, Tehrani KA, Kimpe ND. Binding capacity of brown pigments present in special Spanish sweet wines. Food Sci Technol 2009; 42: 1729-37., 65Wang HY, Qian H, Yao WR. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem 2011; 128: 573-84.
[http://dx.doi.org/10.1016/j.foodchem.2011.03.075] ]. The presence of proteins and carbohydrates in the babassu cake [66Castro AM, Castilho LR, Freire DMG. Characterization of babassu, canola, castor seed and sunflower residual cakes for use as raw materials for fermentation processes. Ind Crops Prod 2016; 83: 140-8.
[http://dx.doi.org/10.1016/j.indcrop.2015.12.050] ] together with the heating during the VBO extraction process contribute to the formation of these compounds.
Regarding antioxidant capacity, both types of babassu oils can be considered low antioxidant power. Ferreira et al. (2011) evaluating the antioxidant capacity of different oils, including babassu oil, obtained comparable results. Essential oils such as oregano and thyme, which have high antioxidant capacity, have values between IC50 3.9 and 1.1 mg/mL [67Viuda-Martos M, Navajas YR, Zapata ES, et al. Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour Fragrance J 2010; 25: 13-9.
[http://dx.doi.org/10.1002/ffj.1951] ]. When compared with excellent antioxidant substances, such as ascorbic acid and BHT [68Cansian RL, Mossi AJ, Oliveira D, et al. Atividade antimicrobiana e antioxidante do óleo essencial de ho-sho (Cinnamomum camphora Ness e Eberm Var. Linaloolifera fujita). Food Sci Technol (Campinas) 2010; 30(2): 378-84.
[http://dx.doi.org/10.1590/S0101-20612010000200014] , 69Ferreira BS, de Almeida CG, Faza LP, et al. Comparative properties of Amazonian oils obtained by different extraction methods. Molecules 2011; 16(7): 5875-85.
[http://dx.doi.org/10.3390/molecules16075875] [PMID: 21750480] ], babassu oil presents activity up to 11.11 x 105 times lower. This may be related to the plant's own anatomy. The babassu kernel is protected within the fruit by at least three layers, epicarp, mesocarp, and endocarp [32Teixeira MA. Babassu–A new approach for an ancient Brazilian biomass. Biomass Bioenergy 2008; 32: 857-64.
[http://dx.doi.org/10.1016/j.biombioe.2007.12.016] , 34Carrazza LR, Ávila JCC, Silva ML. Manual Tecnológico de Aproveitamento Integral do Fruto e da Folha do Babaçu (Attalea spp) 2nd ed. 2012; 68., 70Lorenzi H. Flora brasileira Lorenzi: Arecaceae (palmeiras) 2010; 367.] that form a protective physical barrier for the constituent substances of this almond, so the plant need not produce other compounds for this purpose.
4.2. Contents of Bioactive Compounds
The differences presented by EVBO and VBO oils, both for the antioxidant activity discussed above and the content of phenolic compounds may be related to the higher release of these bioactive substances from the food matrix when it is heated. The presence of water in the cooking step increases the solubility of flavonoids and phenolic acids, consequently increasing their extraction during the process. With the evaporation of water these components remain in the extracted oil. Equivalent results were demonstrated by Serevinatne et al. (2008) in a study about the influence of the method of coconut oil extraction on the content of phenolic compounds of this product [71Seneviratne KN, Dissanayake MSD. Variation of phenolic content in coconut oil extracted by two conventional methods. Int J Food Sci Technol 2008; 43: 597-602.
[http://dx.doi.org/10.1111/j.1365-2621.2006.01493.x] ].
Generally flavonoids, among them, catechin, quercetin and rutin, are the predominant class among the phenolics present in plant foods. However, the presence of phenolic acids, such as gallic and caffeic acids, is considered beneficial since these compounds are considered multipurpose bioactive. These acids, in addition to the known antioxidant effect, may still have antimutagenic, anticarcinogenic, anti-inflammatory and antimicrobial action [59Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects – A review. J Funct Foods 2015; 18: 820-97.
[http://dx.doi.org/10.1016/j.jff.2015.06.018] , 72Xu G, Ye X, Liu D, Ma Y, Chen J. Composition and distribution of phenolic acids in Ponkan (Citrus poonensis Hort. ex Tanaka) and Huyou (Citrus paradisi Macf. Changshanhuyou) during maturity. J Food Compos Anal 2008; 21: 382-9.
[http://dx.doi.org/10.1016/j.jfca.2008.03.003] ].
The tocopherols are part of the compounds with vitamin E activity and are the main antioxidants naturally present in vegetable oils [51Flakelar CL, Prenzler PD, Luckett DJ, Howitt JÁ, Doran G. A rapid method for the simultaneous quantification of the major tocopherols, carotenoids, free and esterified sterols in canola (Brassica napus) oil using normal phase liquid chromatography. Food Chem 2017; 214: 147-55.
[http://dx.doi.org/10.1016/j.foodchem.2016.07.059] [PMID: 2750 7459] ]. The biological activity varies among the isomers, with an increased vitamin activity associated with greater methylation and heightened activity associated with methylation at the fifth carbon, vitamin activity: α > β > γ > δ [6Kerrihard AL, Pegg RB. Utilizing the bioactive contents of specialty oils and fats. Specialty Oils and Fats in Food and Nutrition - Properties, Processing and Applications 2015; 317-48.
[http://dx.doi.org/10.1016/B978-1-78242-376-8.00013-2] , 73Alimentarius C. Codex standard for named vegetable oils (revised, 2015). Codex Standards 2015; 210: 1-14., 74Frank J. Dietary phenolic compounds and vitamin E bioavailability. Model studies in rats and humans. PhD thesis, Swedish University of Agricultural Sciences 2004.]. According to the Specified Oil Standard [73Alimentarius C. Codex standard for named vegetable oils (revised, 2015). Codex Standards 2015; 210: 1-14.] baba ssu oil has low or undetectable levels of these compounds. The results presented in this study show low levels of total tocopherols ( 6.5 mg/100 g of olive oil), and not all of them are found. This fact may be related to the fatty acid composition of babassu oil. Since babassu oil is mostly composed of saturated fatty acids and is therefore more stable, a high concentration of these natural antioxidants is not expected. Other highly saturated vegetable oils, such as coconut oil and palm kernel oil, also have low or undetectable levels of tocopherols [73Alimentarius C. Codex standard for named vegetable oils (revised, 2015). Codex Standards 2015; 210: 1-14.]. On the other hand, in more unsaturated vegetable oils have highly levels of tocopherols, such as palm (
6.5 mg/100 g of olive oil), and not all of them are found. This fact may be related to the fatty acid composition of babassu oil. Since babassu oil is mostly composed of saturated fatty acids and is therefore more stable, a high concentration of these natural antioxidants is not expected. Other highly saturated vegetable oils, such as coconut oil and palm kernel oil, also have low or undetectable levels of tocopherols [73Alimentarius C. Codex standard for named vegetable oils (revised, 2015). Codex Standards 2015; 210: 1-14.]. On the other hand, in more unsaturated vegetable oils have highly levels of tocopherols, such as palm ( 19 mg/100 g oil) [75Kua YL, Gan S, Morris A, Ng HK. A validated, rapid, simple and economical high-performance liquid-chromatography method to quantify palm tocopherol and tocotrienols. J Food Compos Anal 2016; 53: 22-9.
19 mg/100 g oil) [75Kua YL, Gan S, Morris A, Ng HK. A validated, rapid, simple and economical high-performance liquid-chromatography method to quantify palm tocopherol and tocotrienols. J Food Compos Anal 2016; 53: 22-9.
[http://dx.doi.org/10.1016/j.jfca.2016.09.003] ], soybean oil ( 40 mg/100g oil) [76Khan S, Lisa AS, Obaid M, Chowdhury K. Tocopherol content of vegetable oils/ fats and their oxidative deterioration during storage. World J Pharm Pharm Sci 2015; 4(04): 1537-48.], canola (
40 mg/100g oil) [76Khan S, Lisa AS, Obaid M, Chowdhury K. Tocopherol content of vegetable oils/ fats and their oxidative deterioration during storage. World J Pharm Pharm Sci 2015; 4(04): 1537-48.], canola ( 65 mg/100g oil) [77Flakelar CL, Luckett DJ, Howitt JA, Doran G, Prenzler PD. Canola (Brassica napus) oil from Australian cultivars shows promising levels of tocopherols and carotenoids, along with good oxidative stability. J Food Compos Anal 2015; 42: 179-86.
65 mg/100g oil) [77Flakelar CL, Luckett DJ, Howitt JA, Doran G, Prenzler PD. Canola (Brassica napus) oil from Australian cultivars shows promising levels of tocopherols and carotenoids, along with good oxidative stability. J Food Compos Anal 2015; 42: 179-86.
[http://dx.doi.org/10.1016/j.jfca.2015.03.010] ], sunflower ( 80 mg/100g oil) [51Flakelar CL, Prenzler PD, Luckett DJ, Howitt JÁ, Doran G. A rapid method for the simultaneous quantification of the major tocopherols, carotenoids, free and esterified sterols in canola (Brassica napus) oil using normal phase liquid chromatography. Food Chem 2017; 214: 147-55.
80 mg/100g oil) [51Flakelar CL, Prenzler PD, Luckett DJ, Howitt JÁ, Doran G. A rapid method for the simultaneous quantification of the major tocopherols, carotenoids, free and esterified sterols in canola (Brassica napus) oil using normal phase liquid chromatography. Food Chem 2017; 214: 147-55.
[http://dx.doi.org/10.1016/j.foodchem.2016.07.059] [PMID: 2750 7459] ] and olive oil (200-450 mg/100g olive oil) [78Chen H, Angiuli M, Ferrari C, Tombari E, Salvetti G, Bramanti E. Tocopherol speciation as first screening for the assessment of extra virgem olive oil quality by reverse-phase high-performance liquid chromatography/fluorescence detector. Food Chem 2011; 125: 1423-9.
[http://dx.doi.org/10.1016/j.foodchem.2010.10.026] ].
CONCLUSION
The results obtained demonstrate that the extraction method can influence the characteristics and composition of crude babassu oil. Extraction by cooking the crushed kernel cake is capable of incorporating a larger amount of bioactive compounds. This process improves the antioxidant capacity of virgin oil (VBO). Despite this, regardless of the method chosen for its production, babassu oil cannot be considered a potential source of antioxidants.
LIST OF ABBREVIATIONS
| DPPH | = 2,2-diphenyl-1-picrylhydrazyl |
| EVBO | = Extra-Virgin Babassu Oil |
| FCR | = Folin-Ciocalteau Reagent |
| FRAP | = Ferric Reducing Antioxidant Potential |
| HPLC | = High Performance Liquid Chromatography |
| VBO | = Virgin Babassu Oil |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES), Fundação de Amparo a Pesquisa do Estado da Bahia - Brasil (FAPESB), the Instituto Chico Mendes para Conservação da Biodiversidade (ICMBio), the Reserva Extrativista do Extremo Norte do Tocantins (Resex).
REFERENCES
| [1] | USDA - United States Department of Agriculture. Oil Seeds: World Markets and Trad/ September. [updated 2018 Set 22; cited 2018 Set 22] (2018). Available from: https:/ /www.fas. usda. gov/ data/ oilseeds-world-markets-and-trade. |
| [2] | Kumar A, Sharma A, Upadhyaya KC. Vegetable oil: Nutritional and industrial perspective. Curr Genomics 2016; 17(3): 230-40. [http://dx.doi.org/10.2174/1389202917666160202220107] [PMID: 27252590] |
| [3] | Savva SC, Kafatos A. Vegetable oils: Dietary importance. Encyc- lopedia of Food and Health 2016; 365-72. |
| [4] | Hettiarachchi D, Liu Y, Fox B, Sunderland B. Westem Australian sandal wood seed oil: New opportunities. Lipid Technol 2010; 22(2): 27-9. [http://dx.doi.org/10.1002/lite.200900071] |
| [5] | Ergönül PG, Köseoğlu O. Changes in α, β, γ, δ-tocopherol contents of mostly consumed vegetable oils during refining process. CYTA J Food 2014; 12(2): 199-02. [http://dx.doi.org/10.1080/19476337.2013.821672] |
| [6] | Kerrihard AL, Pegg RB. Utilizing the bioactive contents of specialty oils and fats. Specialty Oils and Fats in Food and Nutrition - Properties, Processing and Applications 2015; 317-48. [http://dx.doi.org/10.1016/B978-1-78242-376-8.00013-2] |
| [7] | Ghazani SM, Marangoni AG. Healthy fats and oils 2016. |
| [8] | Sampaio KA, Ayala JV, Van Hoed V, et al. Impact of crude oil quality on the refining conditions and composition of nutraceuticals in refined palm oil. J Food Sci 2017; 82(8): 1842-50. [http://dx.doi.org/10.1111/1750-3841.13805] [PMID: 28722810] |
| [9] | Del Monaco G, Officioso A, D’Angelo S, et al. Characterization of extra virgin olive oils produced with typical Italian varieties by their phenolic profile. Food Chem 2015; 184: 220-8. [http://dx.doi.org/10.1016/j.foodchem.2015.03.071] [PMID: 25872 448] |
| [10] | Oliveira RAD, Neves SC, Ribeiro LM, Lopes PSN, Silvério FO. Storage, oil quality and cryopreservation of babassu palm seeds. Ind Crops Prod 2016; 91: 332-9. [http://dx.doi.org/10.1016/j.indcrop.2016.07.039] |
| [11] | Bauer LC, Damásio JMA, Da Silva MV, Santana DA, Gualberto AS, Simionatto JI. Chemical characterization of pressed and refined licuri (Syagrus coronata) oils. Acta Sci Technol 2013; 35(4): 771-6. [http://dx.doi.org/10.4025/actascitechnol.v35i4.20251] |
| [12] | Waghmare A, Patil S, LeBlanc JG, Sonawane S, Ayra SS. Comparative assessment of algal oil with other vegetable oils for deep frying. Algal Res 2018; 31: 99-106. [http://dx.doi.org/10.1016/j.algal.2018.01.019] |
| [13] | Meinhart AD, da Silveira TFF, de Moraes MR, et al. Optimization of frying oil composition rich in essential fatty acids by mixture design. LWT 2017; 84: 795-803. [http://dx.doi.org/10.1016/j.lwt.2017.06.053] |
| [14] | Guillén MD, Ibargoitia ML, Sopelana P. Margarine: Composition and analysis 2016; 646-53. |
| [15] | Arellano M, Norton IT, Smith P. Specialty oils and fats in margarines and low-fat spreads. Specialty Oils and Fats in Food and Nutrition 2015; 241-70. [http://dx.doi.org/10.1016/B978-1-78242-376-8.00010-7] |
| [16] | Lopes CO, Barcelos MFP, Dias NAA, Carneiro JDS, De Abreu WC. Effect of the addition of spices on reducing the sodium content and increasing the antioxidant activity of margarine. LWT 2014; 58: 63-70. [http://dx.doi.org/10.1016/j.lwt.2014.02.029] |
| [17] | Talbot G. Specialty oils and fats in confectionery. Specialty Oils and Fats in Food and Nutrition 2015; 221-39. [http://dx.doi.org/10.1016/B978-1-78242-376-8.00009-0] |
| [18] | Smith KW. Specialty oils and fats in ice cream. Specialty Oils and Fats in Food and Nutrition 2015; 271-84. [http://dx.doi.org/10.1016/B978-1-78242-376-8.00011-9] |
| [19] | Halim HSA, Selamat J, Mirhosseini SH, Hussain N. Sensory preference and bloom stability of chocolate containing cocoa butter substitute from coconut oil. J Saudi Soc Agric Sci 2018. [http://dx.doi.org/10.1016/j.jssas.2018.02.005] |
| [20] | Pinto F, De Barros DPC, Fonseca LP. Design of multifunctional nanostructured lipid carriers enriched with α-tocopherol using vegetable oils. Ind Crops Prod 2018; 118: 149-59. [http://dx.doi.org/10.1016/j.indcrop.2018.03.042] |
| [21] | Lacatusu I, Arsenie LV, Badea G, Popa O, Oprea O, Badea N. New cosmetic formulations with broad photoprotective and antioxidative activities designed by amaranth and pumpkin seed oils nanocarriers. Ind Crops Prod 2018; 123: 424-33. [http://dx.doi.org/10.1016/j.indcrop.2018.06.083] |
| [22] | Beidokhti MN, Jäger AK. Review of antidiabetic fruits, vegetables, beverages, oils and spices commonly consumed in the diet. J Ethnopharmacol 2017; 201: 26-41. [http://dx.doi.org/10.1016/j.jep.2017.02.031] [PMID: 28257977] |
| [23] | Al-Attar AM, Elnaggar MHR, Almalki EA. Protective effect of some plant oils on diazinon induced hepatorenal toxicity in male rats. Saudi J Biol Sci 2017; 24(6): 1162-71. [http://dx.doi.org/10.1016/j.sjbs.2016.10.013] [PMID: 28855808] |
| [24] | Feranil AB, Duazo PL, Kuzawa CW, Adair LS. Coconut oil is associated with a beneficial lipid profile in pre-menopausal women in the Philippines. Asia Pac J Clin Nutr 2011; 20(2): 190-5. [PMID: 21669587] |
| [25] | Arunima S, Rajamohan T. Influence of virgin coconut oil-enriched diet on the transcriptional regulation of fatty acid synthesis and oxidation in rats - A comparative study. Br J Nutr 2014; 111(10): 1782-90. [http://dx.doi.org/10.1017/S000711451400004X] [PMID: 24513138] |
| [26] | Wei CC, Yu CW, Yen PL, et al. Antioxidant activity, delayed aging, and reduced amyloid-β toxicity of methanol extracts of tea seed pomace from Camellia tenuifolia. J Agric Food Chem 2014; 62(44): 10701-7. [http://dx.doi.org/10.1021/jf503192x] [PMID: 25295856] |
| [27] | Adeyemi KD, Sabow AB, Aghwan ZA, et al. Serum fatty acids, biochemical indices and antioxidant status in goats fed canola oil and palm oil blend. J Anim Sci Technol 2016; 58(6): 6. [http://dx.doi.org/10.1186/s40781-016-0088-2] [PMID: 26858839] |
| [28] | Famurewa AC, Ufebe OG, Egedigwe CA, Nwankwo OE, Obaje GS. Virgin coconut oil supplementation attenuates acute chemotherapy hepatotoxicity induced by anticancer drug methotrexate via inhibition of oxidative stress in rats. Biomed Pharmacother 2017; 87: 437-42. [http://dx.doi.org/10.1016/j.biopha.2016.12.123] [PMID: 28068634] |
| [29] | Wang Y, Niu Y, Zhao X, et al. Fatty acid and phytochemical compositions of plantago seed oils and their functionalities. J Am Oil Chem Soc 2017; 94: 905-12. [http://dx.doi.org/10.1007/s11746-017-3003-1] |
| [30] | Tavakoli J, Emadi T, Hashemi SMB, et al. Chemical properties and oxidative stability of arjan (amygdalus reuteri) kernel oil as emerging edible oil. Food Res Int 2018; 107: 378-84. [http://dx.doi.org/10.1016/j.foodres.2018.02.002] [PMID: 29580498] |
| [31] | Ganesan K, Sukalingam K, Xu B. Impact of consumption and cooking manners of vegetable oils on cardiovascular diseases A critical review. Trends Food Sci Technol 2018; 71: 132-54. [http://dx.doi.org/10.1016/j.tifs.2017.11.003] |
| [32] | Teixeira MA. Babassu–A new approach for an ancient Brazilian biomass. Biomass Bioenergy 2008; 32: 857-64. [http://dx.doi.org/10.1016/j.biombioe.2007.12.016] |
| [33] | IBGE – Instituto Brasileiro de Geografia e Estatística. Produção da Extração Vegetal e da Silvicultura 2015; 30: 1-48. |
| [34] | Carrazza LR, Ávila JCC, Silva ML. Manual Tecnológico de Aproveitamento Integral do Fruto e da Folha do Babaçu (Attalea spp) 2nd ed. 2012; 68. |
| [35] | Soler MP, Vitali AA, Muto EF. Tecnologia de quebra do coco babaçu (Orbignya speciosa). Food Sci Technol (Campinas) 2007; 27(4): 717-22. [http://dx.doi.org/10.1590/S0101-20612007000400007] |
| [36] | Teixeira MA. Heat and power demands in babassu palm oil extraction industry in Brazil. Energy Convers Manage 2005; 46: 2068-74. [http://dx.doi.org/10.1016/j.enconman.2004.10.014] |
| [37] | Da Rós PCM, Costa e Silva W, Grabauskas D, Perez VH, Castro HF. Biodiesel from babassu oil: Characterization of the product obtainedby enzymatic route accelerated by microwave irradiation. Ind Crops Prod 2014; 52: 313-20. [http://dx.doi.org/10.1016/j.indcrop.2013.11.013] |
| [38] | Paiva EJM, da Silva MLCP, Barboza JCS, de Oliveira PC, de Castro HF, Giordani DS. Non-edible babassu oil as a new source for energy production A feasibility transesterification survey assisted by ultrasound. Ultrason Sonochem 2013; 20(3): 833-8. [http://dx.doi.org/10.1016/j.ultsonch.2012.11.003] [PMID: 23207058] |
| [39] | Fonseca FLR, Siqueira JC, Vaz RGMV, et al. Os Benefícios do Babaçu na Alimentação das Aves – Revisão de Literatura 2014; 23. |
| [40] | Albuquerque NI, Contreras CC, Alencar S, et al. Propriedades da carne e perfil de ácidos graxos do pernil de catetos (Tayassu tajacu) alimentados com torta de babaçu (Orbignya phalerata). Arq Bras Med Vet Zootec 2009; 61(6): 1419-27. [http://dx.doi.org/10.1590/S0102-09352009000600023] |
| [41] | Machado GC, Chaves JB, Antoniassi R. Composição em Ácidos Graxos e Caracterização Física e Química de óleos de babaçu. Rev Ceres 2006; 53(308): 463-70. |
| [42] | Medeiros VF, Azevedo ÍM, Carvalho MD, Egito ES, Medeiros AC. Effects of cococonut water and simvastatin in the treatment of sepsis and hemorrhagic shock in rats. Acta Cir Bras 2016; 31(12): 826-33. [http://dx.doi.org/10.1590/s0102-865020160120000008] [PMID: 28076507] |
| [43] | Jubri Z, Latif AA, Top AG, Ngah WZ. Perturbation of cellular immune functions in cigarette smokers and protection by palm oil vitamin E supplementation. Nutr J 2013; 12(1): 2. [http://dx.doi.org/10.1186/1475-2891-12-2] [PMID: 23286246] |
| [44] | Satchithanandam S, Reicks M, Calvert RJ, Cassidy MM, Kritchevsky D. Coconut oil and sesame oil affect lymphatic absorption of cholesterol and fatty acids in rats. J Nutr 1993; 123(11): 1852-8. [http://dx.doi.org/10.1093/jn/123.11.1852] [PMID: 8229300] |
| [45] | Zocchi G. Skin-Feel Agents.Paye AOBM, Maibach HI (Eds), Handbook of Cosmetic Science and Technology, Marcel-Dekker Inc, New York 2001; 357-71. |
| [46] | Montedoro G, Servilli M, Baldioli M, Miniati E. Simple and hydrolyzable phenolic compounds in virgin olive oil. 1. Their extraction, separation, and quantitative and semiquantitative evaluation by HPLC. J Agric Food Chem 1992; 40: 1571-6. [http://dx.doi.org/10.1021/jf00021a019] |
| [47] | Paradiso VM, Clemente A, Summo C, Pasqualone A, Caponio F. Extraction of phenolic compounds from extra virgin olive oil by a natural deep eutectic solvent: Data on UV absorption of the extracts. Data Brief 2016; 8: 553-6. [http://dx.doi.org/10.1016/j.dib.2016.05.076] [PMID: 27504478] |
| [48] | Franco MN, Galeano-Díaz T, López O, et al. Phenolic compounds and antioxidant capacity of virgin olive oil. Food Chem 2014; 163: 289-98. [http://dx.doi.org/10.1016/j.foodchem.2014.04.091] [PMID: 24912 728] |
| [49] | Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem 1996; 239(1): 70-6. [http://dx.doi.org/10.1006/abio.1996.0292] [PMID: 8660627] |
| [50] | Azevedo RSA, Teixeira BS, Sauthier MCS, Santana MVA, Santos WNL, Santana DA. Multivariate analysis of the composition of bioactive in tea of the species Camellia sinensis. Food Chem 2018. [http://dx.doi.org/10.1016/j.foodchem.2018.04.030] [PMID: 3029 2372] |
| [51] | Flakelar CL, Prenzler PD, Luckett DJ, Howitt JÁ, Doran G. A rapid method for the simultaneous quantification of the major tocopherols, carotenoids, free and esterified sterols in canola (Brassica napus) oil using normal phase liquid chromatography. Food Chem 2017; 214: 147-55. [http://dx.doi.org/10.1016/j.foodchem.2016.07.059] [PMID: 2750 7459] |
| [52] | Pyka A, Sliwiok J. Chromatographic separation of tocopherols. J Chromatogr A 2001; 935(1-2): 71-6. [http://dx.doi.org/10.1016/S0021-9673(01)00944-X] [PMID: 11762 786] |
| [53] | Rahmani M, Csallany AS. Chlorophyll and B-carotene pigments in Moroccan virgin olive oils measured by HPLC. J Am Oil Chem Soc 1991; 68: 672-4. [http://dx.doi.org/10.1007/BF02662293] |
| [54] | Tan B, Brzuskiewicz L. Separation of tocopherol and tocotrienol isomers using normal and reverse-phase liquid chromatography. Anal Biochem 1989; 180(2): 368-73. [http://dx.doi.org/10.1016/0003-2697(89)90447-8] [PMID: 2817368] |
| [55] | Apak R, Özyürek M, Güçlü K, Çapanoğlu E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and Electron Transfer (ET)- Based assays. J Agric Food Chem 2016; 64(5): 997-1027. [http://dx.doi.org/10.1021/acs.jafc.5b04739] [PMID: 26728425] |
| [56] | Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K. Methods for testing antioxidant activity. Analyst (Lond) 2002; 127(1): 183-98. [http://dx.doi.org/10.1039/b009171p] [PMID: 11827390] |
| [57] | Castelo-Branco VN, Torres AG. Generalized linear model describes determinants of total antioxidant capacity of refined vegetable oils. Eur J Lipid Sci Technol 2012; 114: 332-42. [http://dx.doi.org/10.1002/ejlt.201100181] |
| [58] | Alves CQ, David JM, David JP, Bahia MV, Aguiar RM. Métodos para determinação de atividade antioxidante in vitro em substratos orgânicos. Quim Nova 2010; 33(10): 2202-10. [http://dx.doi.org/10.1590/S0100-40422010001000033] |
| [59] | Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects – A review. J Funct Foods 2015; 18: 820-97. [http://dx.doi.org/10.1016/j.jff.2015.06.018] |
| [60] | Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 2005; 53(10): 4290-302. [http://dx.doi.org/10.1021/jf0502698] [PMID: 15884874] |
| [61] | McClemenets DJ, Decker EA. Lipids Fenema’s food chemistry 2007; 155-216. |
| [62] | Mesias M, Delgado-Andrade C. Melanoidins as potential functional food ingradient. Curr Opin Food Sci 2017; 14: 37-42. [http://dx.doi.org/10.1016/j.cofs.2017.01.007] |
| [63] | D. J., & Huber, E. A.Carbohydrates Fenema’s food chemistry 2007; 75-130. |
| [64] | Ramonaityte DT, Kersiene M, Adams A, Tehrani KA, Kimpe ND. Binding capacity of brown pigments present in special Spanish sweet wines. Food Sci Technol 2009; 42: 1729-37. |
| [65] | Wang HY, Qian H, Yao WR. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem 2011; 128: 573-84. [http://dx.doi.org/10.1016/j.foodchem.2011.03.075] |
| [66] | Castro AM, Castilho LR, Freire DMG. Characterization of babassu, canola, castor seed and sunflower residual cakes for use as raw materials for fermentation processes. Ind Crops Prod 2016; 83: 140-8. [http://dx.doi.org/10.1016/j.indcrop.2015.12.050] |
| [67] | Viuda-Martos M, Navajas YR, Zapata ES, et al. Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour Fragrance J 2010; 25: 13-9. [http://dx.doi.org/10.1002/ffj.1951] |
| [68] | Cansian RL, Mossi AJ, Oliveira D, et al. Atividade antimicrobiana e antioxidante do óleo essencial de ho-sho (Cinnamomum camphora Ness e Eberm Var. Linaloolifera fujita). Food Sci Technol (Campinas) 2010; 30(2): 378-84. [http://dx.doi.org/10.1590/S0101-20612010000200014] |
| [69] | Ferreira BS, de Almeida CG, Faza LP, et al. Comparative properties of Amazonian oils obtained by different extraction methods. Molecules 2011; 16(7): 5875-85. [http://dx.doi.org/10.3390/molecules16075875] [PMID: 21750480] |
| [70] | Lorenzi H. Flora brasileira Lorenzi: Arecaceae (palmeiras) 2010; 367. |
| [71] | Seneviratne KN, Dissanayake MSD. Variation of phenolic content in coconut oil extracted by two conventional methods. Int J Food Sci Technol 2008; 43: 597-602. [http://dx.doi.org/10.1111/j.1365-2621.2006.01493.x] |
| [72] | Xu G, Ye X, Liu D, Ma Y, Chen J. Composition and distribution of phenolic acids in Ponkan (Citrus poonensis Hort. ex Tanaka) and Huyou (Citrus paradisi Macf. Changshanhuyou) during maturity. J Food Compos Anal 2008; 21: 382-9. [http://dx.doi.org/10.1016/j.jfca.2008.03.003] |
| [73] | Alimentarius C. Codex standard for named vegetable oils (revised, 2015). Codex Standards 2015; 210: 1-14. |
| [74] | Frank J. Dietary phenolic compounds and vitamin E bioavailability. Model studies in rats and humans. PhD thesis, Swedish University of Agricultural Sciences 2004. |
| [75] | Kua YL, Gan S, Morris A, Ng HK. A validated, rapid, simple and economical high-performance liquid-chromatography method to quantify palm tocopherol and tocotrienols. J Food Compos Anal 2016; 53: 22-9. [http://dx.doi.org/10.1016/j.jfca.2016.09.003] |
| [76] | Khan S, Lisa AS, Obaid M, Chowdhury K. Tocopherol content of vegetable oils/ fats and their oxidative deterioration during storage. World J Pharm Pharm Sci 2015; 4(04): 1537-48. |
| [77] | Flakelar CL, Luckett DJ, Howitt JA, Doran G, Prenzler PD. Canola (Brassica napus) oil from Australian cultivars shows promising levels of tocopherols and carotenoids, along with good oxidative stability. J Food Compos Anal 2015; 42: 179-86. [http://dx.doi.org/10.1016/j.jfca.2015.03.010] |
| [78] | Chen H, Angiuli M, Ferrari C, Tombari E, Salvetti G, Bramanti E. Tocopherol speciation as first screening for the assessment of extra virgem olive oil quality by reverse-phase high-performance liquid chromatography/fluorescence detector. Food Chem 2011; 125: 1423-9. [http://dx.doi.org/10.1016/j.foodchem.2010.10.026] |