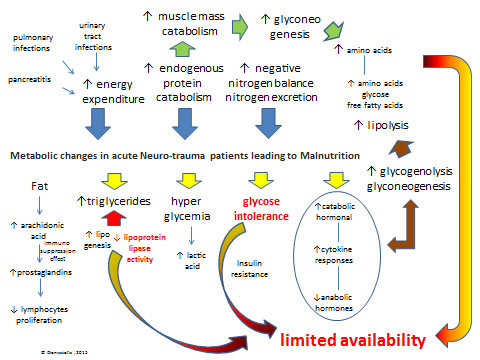
Fig. (2) Metabolic changes in acute Neurotrauma patients leading to Malnutrition: There is a dramatic increase (see blue arrows) in energy expenditure, endogenous protein catabolism and nitrogen excretion after lesion-injury. Primary metabolic changes include an hormonal cataract (see yellow arrows) with elevated catabolic hormonal and cytokine responses in blood serum (i.e. cortisol, catecholamines, and glucagon) and peripheral-tissue resistance to endogenous anabolic hormones (i.e. insulin and insulin-like growth factor 1) and increased blood and tissue levels of proinflammatory cytokines (i.e. interleukin-1, interleukin-6, interleukin-8, and tumor necrosis factor α), respectively. Glucose intolerance may be caused by hyper metabolic-catabolic stress response, administration of steroids, the parenteral/enteral nutrition, and atrophy as a consequence of aponeurosis which results in gluconeogenesis. Hyperglycemia follows head injury or spinal cord increase the production of lactic acid, which may have the reverse effect on neurological recovery from injury. Unfortunately, increases in glycogenolysis and gluconeogenesis, is enhancing lipolysis, which provides endogenous glucose, amino acids, and free fatty acids (see brown arrows) that are required for cellular and organ function and wound healing but are insufficient to meet metabolic needs due to limited availability for use by peripheral tissues because of insulin resistance and inhibition of lipoprotein lipase (see limited availability in red). Another serious metabolic issue is negative nitrogen balance, due to excessive secretion of nitrogen because of protein use (mainly due to pulmonary infections or urinary tract infractions, and pancreatitis).