- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Nutrition Journal
(Discontinued)
ISSN: 1874-2882 ― Volume 15, 2021
A Rapid and Simple Method for Fatty Acid Profiling and Determination of ω-3 Index in Red Blood Cells
Olufunmilola Akinyemi1, Geza Bruckner1, John Johnson3, Terry A. Lennie2, David Hildebrand3, *
Abstract
Fatty acid profiling has become a very useful and effective tool in the diagnosis, prevention and treatment of several diseases with cardiovascular disease being particularly important. In order to arrive at accurate conclusions that would help promote the health of individuals plagued by such diseases, not only excellent laboratory methods are required, but also very important monitoring responses to treatment. Improvements in methods of fatty acid profiling in biological systems regarding safety of extraction, precision and time for analysis are valuable. The ω-3 index (a measure of the amount of eicosapentaenoic acid, EPA, and docosahexaenoic acid, DHA, in Red Blood Cell membranes expressed as the percent of total fatty acids) is of growing interest because it has been reported to provide prognostic information regarding the risk for heart diseases. Sodium methoxide has been widely used for the determination of ω -3 fatty acids in food samples. This study demonstrates that sodium methoxide can be used effectively in RBC fatty acid profiling and determination of the ω-3 index. Briefly, the fatty acid profiles and ω-3 index of red blood cell samples were analyzed and results compared using three different methods: a two- step extraction and methylation method described by Hara and Radin, a single step extraction and methylation method described by Harris et al. and the sodium methoxide method.
Our results revealed that there were no statistically significant differences (p<0.05) between the three methods for the representative fatty acids, [16:0 (p = 0.10), 18:0 (p=0.40), 18:1(ω9) (p = 0.29), 18:2(ω6) (p = 0.95), 18:3(ω3) (p = 0.50), 20:5(ω3) (p=0.56), 22:6(ω3) (p = 0.06)] and ω-3 index (p = 0.11) except for 20:4(ω6), (P = 0.02). In conclusion, we show that sodium methoxide can be used effectively in a one-step extraction and methylation procedure for high throughput analysis of fatty acids in red blood cell membranes. It is rapid (10 minute extraction and methylation), simple, safer than and as accurate as other commonly reported methods.
Article Information
Identifiers and Pagination:
Year: 2017Volume: 11
First Page: 17
Last Page: 26
Publisher Id: TONUTRJ-11-17
DOI: 10.2174/1874288201711010017
Article History:
Received Date: 15/07/2016Revision Received Date: 23/12/2016
Acceptance Date: 25/12/2016
Electronic publication date: 31/01/2017
Collection year: 2017
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Department of Plant & Soil Sciences, University of Kentucky, Lexington, KY 403 PSB, USA; Tel: 859/218-0760, Fax: 8592577848; E-mail: dhild@uky.edu
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 15-07-2016 |
Original Manuscript | A Rapid and Simple Method for Fatty Acid Profiling and Determination of ω-3 Index in Red Blood Cells | |
INTRODUCTION
Fatty acids constitute an important structural element of biological membranes, serving many vital functions in biological systems such as providing energy sources and acting as signaling molecules [1Liu RL, Zhang J, Mou ZL, Hao SL, Zhang ZQ. Microwave-assisted one-step extraction-derivatization for rapid analysis of fatty acids profile in herbal medicine by gas chromatography-mass spectrometry. Analyst (Lond) 2012; 137(21): 5135-43.
[http://dx.doi.org/10.1039/c2an36178g] [PMID: 22968083] ].
The fatty acid composition of the red blood cell membrane has been reported to be an invaluable tool in providing prognostic information regarding the risk for coronary heart disease, cancers, neuropsychiatric diseases, accelerated cellular aging and early mortality [2Harris WS, Pottala JV, Varvel SA, Borowski JJ, Ward JN, McConnell JP. Erythrocyte omega-3 fatty acids increase and linoleic acid decreases with age: observations from 160,000 patients. Prostaglandins Leukot Essent Fatty Acids 2013; 88(4): 257-63.
[http://dx.doi.org/10.1016/j.plefa.2012.12.004] [PMID: 23375840] -7Roy S, Brasky TM, Belury MA, et al. Associations of erythrocyte ω-3 fatty acids with biomarkers of ω-3 fatty acids and inflammation in breast tissue. Int J Cancer 2015; 137(12): 2934-46.
[http://dx.doi.org/10.1002/ijc.29675] [PMID: 26137879] ].
Of growing interest is the omega-3 Index (a measure of the amount of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in Red Blood Cell membranes expressed as the percent of total fatty acids) which has been shown to be inversely proportional to the risk for sudden cardiac death [2Harris WS, Pottala JV, Varvel SA, Borowski JJ, Ward JN, McConnell JP. Erythrocyte omega-3 fatty acids increase and linoleic acid decreases with age: observations from 160,000 patients. Prostaglandins Leukot Essent Fatty Acids 2013; 88(4): 257-63.
[http://dx.doi.org/10.1016/j.plefa.2012.12.004] [PMID: 23375840] , 8Harris WS. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr 2008; 87(6): 1997S-2002S.
[PMID: 18541601] , 9Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med 2004; 39(1): 212-20.
[http://dx.doi.org/10.1016/j.ypmed.2004.02.030] [PMID: 15208005] ]. This has led to the increased awareness of the health benefits of foods rich in EPA and DHA with an ultimate aim of increasing blood levels of these fatty acids and hence increasing the omega-3 Index [10Harris WS, Pottala JV, Vasan RS, Larson MG, Robins SJ. Changes in erythrocyte membrane trans and marine fatty acids between 1999 and 2006 in older Americans. J Nutr 2012; 142(7): 1297-303.
[http://dx.doi.org/10.3945/jn.112.158295] [PMID: 22623386] , 11Von Schacky C. Omega-3 fatty acids vs. cardiac diseasethe contribution of the omega-3 index. Cell Mol Biol (Noisy-le-grand) 2010; 56(1): 93-101.
[PMID: 20196973] ].
Without a full knowledge of the total fatty acid composition of Red Blood Cell membranes, it would be almost impossible to calculate the omega-3 index. This requires the need for efficient laboratory methods that would enable high throughput analysis of fatty acids in biological systems.
FATTY ACID COMPOSITION IN BIOLOGICAL SYSTEMS
Studies determining the fatty acid composition of a myriad of biological samples have previously been reported. Samples studied for example include: skin, breast milk, sperm, cheek cells, monocytes, neutrophils, T-lymphocytes, B-lymphocytes, red blood cells and whole blood [12Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008; 47(5): 348-80.
[http://dx.doi.org/10.1016/j.plipres.2008.03.003] [PMID: 18435934] -16Klingler M, Klem S, Demmelmair H, Koletzko B. Comparison of the incorporation of orally administered DHA into plasma, erythrocyte and cheek cell glycerophospholipids. Br J Nutr 2013; 109(5): 962-8.
[http://dx.doi.org/10.1017/S000711451200222X] [PMID: 22874641] ]. However, the most often reported biological samples used in determination of fatty acid composition in relation to nutritional status are adipose tissue, plasma, platelets and erythrocytes [12Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008; 47(5): 348-80.
[http://dx.doi.org/10.1016/j.plipres.2008.03.003] [PMID: 18435934] ].
A vivid picture of the long-term dietary intake, with particular emphasis on long-chain omega-3 polyunsaturated fatty acids, can be obtained by deducing the concentrations of fatty acids in red blood cells (RBCs) [17Clayton EH, Gulliver CE, Piltz JW, Taylor RD, Blake RJ, Meyer RG. Improved extraction of saturated fatty acids but not omega-3 fatty acids from sheep red blood cells using a one-step extraction procedure. Lipids 2012; 47(7): 719-27.
[http://dx.doi.org/10.1007/s11745-012-3674-1] [PMID: 22570172] , 18Harris WS, Thomas RM. Biological variability of blood omega-3 biomarkers. Clin Biochem 2010; 43(3): 338-40.
[http://dx.doi.org/10.1016/j.clinbiochem.2009.08.016] [PMID: 19733159] ]. The fatty acids present in erythrocyte membrane phospholipids can be incorporated during de novo synthesis or via exchange from the pool of fatty acids in the plasma [12Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008; 47(5): 348-80.
[http://dx.doi.org/10.1016/j.plipres.2008.03.003] [PMID: 18435934] ]. The membrane fatty acid composition is a dynamic system, one that keeps changing based on selective recruitment of fatty acids from precursor pools [19Tepsic J, Vucic V, Arsic A, Blazencic-Mladenovic V, Mazic S, Glibetic M. Plasma and erythrocyte phospholipid fatty acid profile in professional basketball and football players. Eur J Appl Physiol 2009; 107(3): 359-65.
[http://dx.doi.org/10.1007/s00421-009-1131-5] [PMID: 19633987] ]. The fatty acid composition of plasma phospholipids poorly reflect long-term dietary intake (as a result of the fast turnover) while those of red blood cells portray dietary fat intake during the preceding months.
The fatty acid composition of phospholipids contributes considerably to membrane properties and also, is peculiar to which class it belongs [12Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008; 47(5): 348-80.
[http://dx.doi.org/10.1016/j.plipres.2008.03.003] [PMID: 18435934] ]. Studies have been carried out to determine the total erythrocyte membrane fatty acids as well as the proportions that make up each phospholipid class. Agren et al. [20Agren JJ, Törmälä ML, Nenonen MT, Hänninen OO. Fatty acid composition of erythrocyte, platelet, and serum lipids in strict vegans. Lipids 1995; 30(4): 365-9.
[http://dx.doi.org/10.1007/BF02536047] [PMID: 7609607] ] and Hodson et al. [12Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008; 47(5): 348-80.
[http://dx.doi.org/10.1016/j.plipres.2008.03.003] [PMID: 18435934] ] reported data showing the percentages of fatty acids that comprise the erythrocyte membranes and phospholipid classes (Table 1).
The data presented in Table 1 are based on results from two studies [12Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008; 47(5): 348-80.
[http://dx.doi.org/10.1016/j.plipres.2008.03.003] [PMID: 18435934] , 20Agren JJ, Törmälä ML, Nenonen MT, Hänninen OO. Fatty acid composition of erythrocyte, platelet, and serum lipids in strict vegans. Lipids 1995; 30(4): 365-9.
[http://dx.doi.org/10.1007/BF02536047] [PMID: 7609607] ]. The amounts reported for total erythrocyte membrane fatty acids from the Hodson et al. study are comparable to those from Agren et al. There are similar amounts of total fatty acids 16:0, 18:0, 18:1 n-9, 18:2 n-6, 18:3 n-3, 20:5 n-3 with 16:0 being the most abundant fatty acid. When comparing total erythrocyte fatty acid composition with the fractions PE, PC and PS, there are some remarkable differences. PE has a lower abundance of 16:0, 18:0, 18:2 n-6 and 20:3 n-6 compared to the total fatty acid composition. PC has higher abundance of 16:0 and 18:2 n-6. These data are in agreement with other reported studies [21Dougherty RM, Galli C, Ferro-Luzzi A, Iacono JM. Lipid and phospholipid fatty acid composition of plasma, red blood cells, and platelets and how they are affected by dietary lipids: a study of normal subjects from Italy, Finland, and the USA. Am J Clin Nutr 1987; 45(2): 443-55.
[PMID: 3812343] -24Prisco D, Filippini M, Francalanci I, et al. Effect of n-3 polyunsaturated fatty acid intake on phospholipid fatty acid composition in plasma and erythrocytes. Am J Clin Nutr 1996; 63(6): 925-32.
[PMID: 8644688] ].
METHODS OF DETERMINING FATTY ACID COMPOSITION
Scientists have worked extensively to improve laboratory methods used to measure the fatty acid content of biological samples with a goal to developing newer methods that would; (i) improve the extraction and derivatization efficiency of lipids, (ii) be less time-consuming, (iii) cost-effective and, (iv) most importantly be safe.
Conventionally, fatty acid analysis involves procedures that consist of an initial extraction step, followed by derivatization of fatty acids (FAs) to fatty acid methyl esters (FAMEs), and subsequent chromatographic determination of FAMEs (1).
Previous studies have shown that fatty acid analysis of human red blood cell can be determined by a two-step extraction and methylation or a combined one-step extraction and methylation procedure [17Clayton EH, Gulliver CE, Piltz JW, Taylor RD, Blake RJ, Meyer RG. Improved extraction of saturated fatty acids but not omega-3 fatty acids from sheep red blood cells using a one-step extraction procedure. Lipids 2012; 47(7): 719-27.
[http://dx.doi.org/10.1007/s11745-012-3674-1] [PMID: 22570172] , 20Agren JJ, Törmälä ML, Nenonen MT, Hänninen OO. Fatty acid composition of erythrocyte, platelet, and serum lipids in strict vegans. Lipids 1995; 30(4): 365-9.
[http://dx.doi.org/10.1007/BF02536047] [PMID: 7609607] , 25Magnusardottir AR, Steingrimsdottir L, Thorgeirsdottir H, Gunnlaugsson G, Skuladottir GV. Docosahexaenoic acid in red blood cells of women of reproductive age is positively associated with oral contraceptive use and physical activity. Prostaglandins Leukot Essent Fatty Acids 2009; 80(1): 27-32.
[http://dx.doi.org/10.1016/j.plefa.2008.10.004] [PMID: 19071003] , 26Eder K. Gas chromatographic analysis of fatty acid methyl esters. J Chromatogr B Biomed Appl 1995; 671(1-2): 113-31.
[http://dx.doi.org/10.1016/0378-4347(95)00142-6] [PMID: 8520689] ].
METHYLATION PROCEDURES
Several different methylating agents have been used in determining the fatty acid composition of RBC. Of notable interest is the use of 14% boron trifluoride/methanol which is widely used in the transmethylation of fatty acids in red blood cells [1Liu RL, Zhang J, Mou ZL, Hao SL, Zhang ZQ. Microwave-assisted one-step extraction-derivatization for rapid analysis of fatty acids profile in herbal medicine by gas chromatography-mass spectrometry. Analyst (Lond) 2012; 137(21): 5135-43.
[http://dx.doi.org/10.1039/c2an36178g] [PMID: 22968083] , 17Clayton EH, Gulliver CE, Piltz JW, Taylor RD, Blake RJ, Meyer RG. Improved extraction of saturated fatty acids but not omega-3 fatty acids from sheep red blood cells using a one-step extraction procedure. Lipids 2012; 47(7): 719-27.
[http://dx.doi.org/10.1007/s11745-012-3674-1] [PMID: 22570172] , 27Otsuka R, Kato Y, Imai T, Ando F, Shimokata H. Higher serum EPA or DHA, and lower ARA compositions with age independent fatty acid intake in Japanese aged 40 to 79. Lipids 2013; 48(7): 719-27.
[http://dx.doi.org/10.1007/s11745-013-3763-9] [PMID: 23389403] , 28Shantha NC, Napolitano GE. Gas chromatography of fatty acids. J Chromatogr A 1992; 624(1-2): 37-51.
[http://dx.doi.org/10.1016/0021-9673(92)85673-H] [PMID: 1494015] ]. Other methylating reagents used include acetyl chloride [29Rodríguez-Palmero M, Lopez-Sabater MC, Castellote-Bargallo AI, De la Torre-Boronat MC, Rivero-Urgell M. Comparison of two methods for the determination of fatty acid profiles in plasma and erythrocytes. J Chromatogr A 1998; 793(2): 435-40.
[http://dx.doi.org/10.1016/S0021-9673(97)10554-4] [PMID: 9474792] ], 2% sulfuric acid in methanol [30Di Marino L, Maffettone A, Cipriano P, et al. Is the erythrocyte membrane fatty acid composition a valid index of skeletal muscle membrane fatty acid composition? Metabolism 2000; 49(9): 1164-6.
[http://dx.doi.org/10.1053/meta.2000.8616] [PMID: 11016898] ] and sodium methoxide [16Klingler M, Klem S, Demmelmair H, Koletzko B. Comparison of the incorporation of orally administered DHA into plasma, erythrocyte and cheek cell glycerophospholipids. Br J Nutr 2013; 109(5): 962-8.
[http://dx.doi.org/10.1017/S000711451200222X] [PMID: 22874641] , 31Laryea MD, Cieslicki P, Diekmann E, Wendel U. Analysis of the fatty acid composition of erythrocyte phospholipids by a base catalysed transe sterification method prevention of formation of dimethylacetals. Clin Chim Acta 1988; 171(1): 11-8.
[http://dx.doi.org/10.1016/0009-8981(88)90286-0] [PMID: 3349633] ].
In recent years, various studies have suggested that a combined one-step extraction and methylation procedure is faster and simpler in many ways when compared to the conventional method. Kang and Wang described a simplified method for analysis of polyunsaturated fatty acids in which they compared the conventional two-step method (prior extraction with chloroform/methanol and subsequent methylation with 14% boron trifluoride/methanol) to a combined extraction-methylation procedure. It was concluded from his study that prior lipid extraction in lipid analysis can be omitted without affecting the recovery of long chain FAs [32Kang JX, Wang J. A simplified method for analysis of polyunsaturated fatty acids. BMC Biochem 2005; 6: 5.
[http://dx.doi.org/10.1186/1471-2091-6-5] [PMID: 15790399] ]. K. Eder, in his review, stated that direct trans-esterification proceeds at a faster rate than saponification, with hydrolysis and esterification taking place in a single step, requiring only one reagent [26Eder K. Gas chromatographic analysis of fatty acid methyl esters. J Chromatogr B Biomed Appl 1995; 671(1-2): 113-31.
[http://dx.doi.org/10.1016/0378-4347(95)00142-6] [PMID: 8520689] ]. However, accuracy of any laboratory method should be checked with the use of primary lipid standards prior to the analysis of biological samples. Incomplete recovery of FAMEs from lipid standards would necessitate optimization of analytical conditions before trans-esterification of the sample [26Eder K. Gas chromatographic analysis of fatty acid methyl esters. J Chromatogr B Biomed Appl 1995; 671(1-2): 113-31.
[http://dx.doi.org/10.1016/0378-4347(95)00142-6] [PMID: 8520689] ].
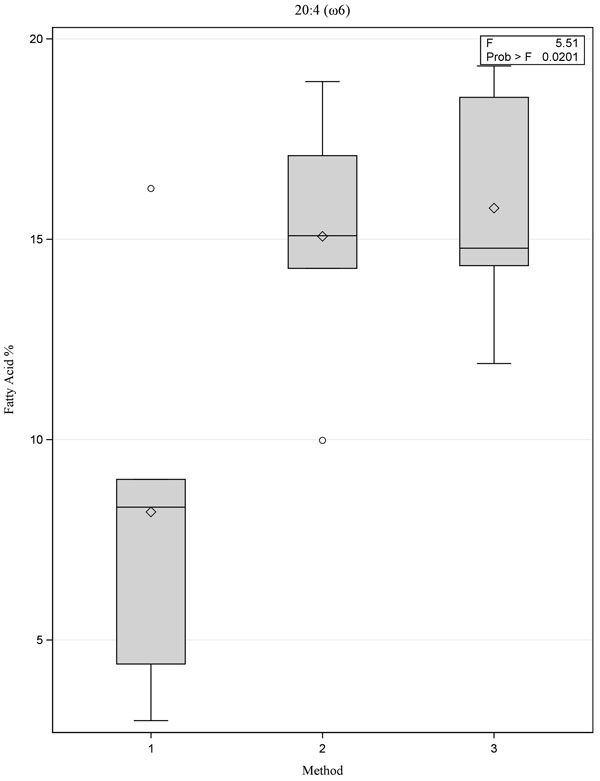 |
Fig. (1) Box plot showing comparison of fatty acid 20:4 (ω6) for the three methods, p=0.02. |
Direct trans-esterification of lipids can either be acid or base-catalyzed. Reagents commonly used for acid catalyzed trans-esterification are: methanolic hydrochloric and sulfuric acid [33Bell JG, Mackinlay EE, Dick JR, Younger I, Lands B, Gilhooly T. Using a fingertip whole blood sample for rapid fatty acid measurement: method validation and correlation with erythrocyte polar lipid compositions in UK subjects. Br J Nutr 2011; 106(9): 1408-15.
[http://dx.doi.org/10.1017/S0007114511001978] [PMID: 21736805] ]; and boron trifluoride in methanol [10Harris WS, Pottala JV, Vasan RS, Larson MG, Robins SJ. Changes in erythrocyte membrane trans and marine fatty acids between 1999 and 2006 in older Americans. J Nutr 2012; 142(7): 1297-303.
[http://dx.doi.org/10.3945/jn.112.158295] [PMID: 22623386] , 34Coviello G, Tutino V, Notarnicola M, Caruso MG. Erythrocyte membrane fatty acids profile in colorectal cancer patients: a preliminary study. Anticancer Res 2014; 34(9): 4775-9.
[PMID: 25202057] , 35Koehrer P, Saab S, Berdeaux O, et al. Erythrocyte phospholipid and polyunsaturated fatty acid composition in diabetic retinopathy. PLoS One 2014; 9(9): e106912.
[http://dx.doi.org/10.1371/journal.pone.0106912] [PMID: 25188352] ] whereas reagents that have been used for base-catalyzed trans-esterification of biological samples are sodium methoxide [16Klingler M, Klem S, Demmelmair H, Koletzko B. Comparison of the incorporation of orally administered DHA into plasma, erythrocyte and cheek cell glycerophospholipids. Br J Nutr 2013; 109(5): 962-8.
[http://dx.doi.org/10.1017/S000711451200222X] [PMID: 22874641] , 31Laryea MD, Cieslicki P, Diekmann E, Wendel U. Analysis of the fatty acid composition of erythrocyte phospholipids by a base catalysed transe sterification method prevention of formation of dimethylacetals. Clin Chim Acta 1988; 171(1): 11-8.
[http://dx.doi.org/10.1016/0009-8981(88)90286-0] [PMID: 3349633] ] and potassium hydroxide in methanol [6Puca AA, Andrew P, Novelli V, et al. Fatty acid profile of erythrocyte membranes as possible biomarker of longevity. Rejuvenation Res 2008; 11(1): 63-72.
[http://dx.doi.org/10.1089/rej.2007.0566] [PMID: 18160025] ]. Boron trifluoride in methanol (12-14%) is the most often used for transesterification of RBC lipids [26Eder K. Gas chromatographic analysis of fatty acid methyl esters. J Chromatogr B Biomed Appl 1995; 671(1-2): 113-31.
[http://dx.doi.org/10.1016/0378-4347(95)00142-6] [PMID: 8520689] ]. Several other reagents used for trans-esterification of human and plant samples include acetyl chloride [17Clayton EH, Gulliver CE, Piltz JW, Taylor RD, Blake RJ, Meyer RG. Improved extraction of saturated fatty acids but not omega-3 fatty acids from sheep red blood cells using a one-step extraction procedure. Lipids 2012; 47(7): 719-27.
[http://dx.doi.org/10.1007/s11745-012-3674-1] [PMID: 22570172] , 26Eder K. Gas chromatographic analysis of fatty acid methyl esters. J Chromatogr B Biomed Appl 1995; 671(1-2): 113-31.
[http://dx.doi.org/10.1016/0378-4347(95)00142-6] [PMID: 8520689] , 36Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 1986; 27(1): 114-20.
[PMID: 3958609] , 37Lin YH, Hanson JA, Strandjord SE, et al. Fast transmethylation of total lipids in dried blood by microwave irradiation and its application to a population study. Lipids 2014; 49(8): 839-51.
[http://dx.doi.org/10.1007/s11745-014-3918-3] [PMID: 24986160] ] and aluminum chloride [26Eder K. Gas chromatographic analysis of fatty acid methyl esters. J Chromatogr B Biomed Appl 1995; 671(1-2): 113-31.
[http://dx.doi.org/10.1016/0378-4347(95)00142-6] [PMID: 8520689] , 38Segura R. Preparation of fatty acid methyl esters by direct transesterification of lipids with aluminium chloride-methanol. J Chromatogr A 1988; 441(1): 99-113.
[http://dx.doi.org/10.1016/S0021-9673(01)84658-6] [PMID: 3403681] ].
The aim of this study was to determine if the sodium methoxide method can be used effectively in a one-step extraction and methylation procedure for high throughput analysis of fatty acids in red blood cell membranes when compared with the notable Hara and Radin [39Hara A, Radin NS. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 1978; 90(1): 420-6.
[http://dx.doi.org/10.1016/0003-2697(78)90046-5] [PMID: 727482] ] and the Harris et al. method [10Harris WS, Pottala JV, Vasan RS, Larson MG, Robins SJ. Changes in erythrocyte membrane trans and marine fatty acids between 1999 and 2006 in older Americans. J Nutr 2012; 142(7): 1297-303.
[http://dx.doi.org/10.3945/jn.112.158295] [PMID: 22623386] ].
MATERIALS AND METHODS
Materials
- FAME standard mixtures were obtained from Nu-Chek Prep, Inc. of Elysian, MN. Standard mixtures used were: GLC-538, GLC-455, GLC-480 and O8C. Standards were prepared by initially dissolving them in chloroform followed by serial dilution with isooctane. The selected GC conditions were sufficient to provide baseline resolution of all components of the above standard mixtures except for 24:0, 22:3n3 (co-elution) and 22:4n6 (partial resolution).
- Sodium methoxide prepared in our lab. Briefly, stock solution was prepared by dissolving 18 g of sodium (stored under toluene) in 1L of methanol. Care was taken to avoid moisture contamination due to the ability of sodium metal to react exothermically with water to produce sodium hydroxide and the flammable hydrogen gas.
- Methanol containing 14% boron trifluoride, hexane (Fisher Scientific).
Laboratory Methods
Whole blood was centrifuged at 1,300 g for 10 minutes. Red blood cells (RBC) were isolated, washed twice with phosphate buffered saline and aliquoted into 10 centrifuge tubes (0.5 mL each). 100µL of 2.5% BHT (Butylated hydroxytoluene) was added, for each mL of blood, as an antioxidant. Thereafter, the packed red blood cells were stored under argon gas at -80°C prior to analysis. The fatty acid profiles as well as the ω-3 index of the red blood cells were determined and compared using three methods.
Method 1: The Hara and Radin Method (Hara and Radin, 1978; (39-41) (Two-Step Extraction and Methylation)
Extraction was accomplished using 3:2 hexane isopropanol and the subsequent methylation carried out with 14% boron trifluoride in methanol*. Briefly, to 50-µL of RBC*, 2mL of hexane:isopropanol followed by 2mL of hexane was added. The mixtures were vortexed well and upper hexane layer was transferred to screw capped tubes. Volume of upper hexane layer was reduced to ≤ 0.25mL by blowing with nitrogen gas. Methylation was carried out as in Method 2 with 14% boron trifluoride in methanol.
Method 2: The Described Method of Harris et al. (10) (One-Step Extraction and Methylation)
14% boron trifluoride was used*.
To 50-µL RBC* in screw capped tubes, 250-µL 14% boron trifluoride in methanol followed by 250-µL hexane was added. Tubes were flushed with argon and sealed. Mixture was briefly vortexed and heated at 100°C for 10 minutes. After cooling, 2mL isooctane + 0.001% BHT was added and vortexed. Thereafter, upper isooctane layer was transferred into GC vials for analysis.
Method 3: Sodium Methoxide Method (One-Step Extraction and Methylation)
To 50-µL aliquot of RBC* in open glass tubes, 0.5 mL sodium methoxide was added. The mixtures were vortexed for about 10-20 seconds and placed on a shaker for 10 minutes. Thereafter, 2 mL isooctane + 0.001% BHT was added and then vortexed well. The upper isooctane layer was transferred into GC vials for analysis.
Fatty Acid Composition Analysis
FAMEs were analyzed using a Varian 3800 Gas Chromatograph equipped with a model 1177 split/splitless injector and FID detector. The injector temperature was 240°C and the detector temperature was 260°C. Sample volumes of between 1.5µL and 2.0µL were injected in splitless mode using a Varian model 8400 autoinjector. The chromatographic column used was an Agilent VF-23ms of length 30m, inside diameter of 0.25mm and a film thickness of 0.25 microns. The carrier gas was hydrogen held at a constant flow of 1.6 mL/min using electronic flow control. The column oven temperature profile was: 80°C initial hold for 1.0 min, then programmed to 120°C at 10°C/min, then to 204°C at 4°C/min and then to 250°C at 20°C/min with a final hold of 7 min (total run time of 35.3 min). The total run time was sufficient to elute large sterol peaks. Data were compared by peak area against the peak area of the standard mixture of fatty acids characteristic of RBCs. Results are expressed as means ± SE.
Calculation of the ω3 index: Amounts of EPA + DHA/Total fatty acids x 100%.
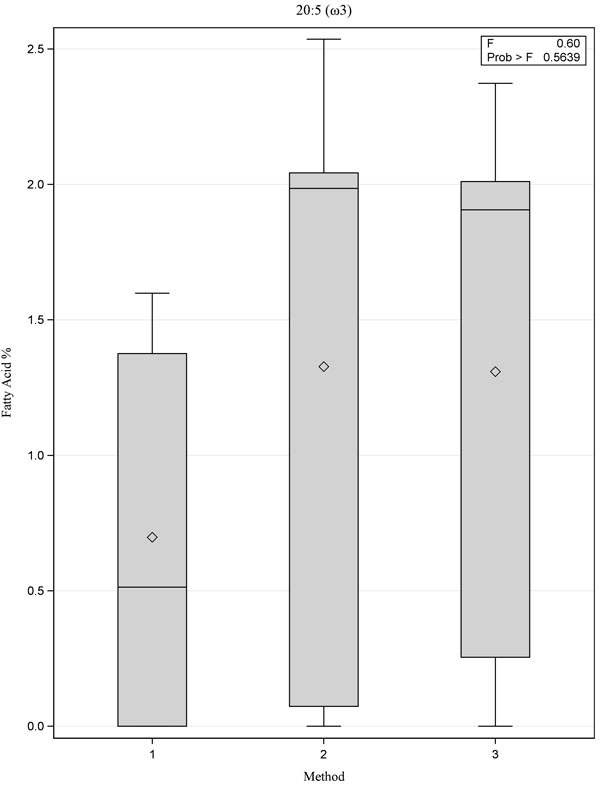 |
Fig. (2) Box plot showing comparison of fatty acid 20:5 (ω3) for the three methods, p=0.56. |
STATISTICAL ANALYSIS
Statistical analysis to determine differences between methods was investigated using SAS version 9.4 by subjecting collected data to analysis of variance (ANOVA) and two-sample t-tests were used to compare the means. P values of < 0.05 were considered significant.
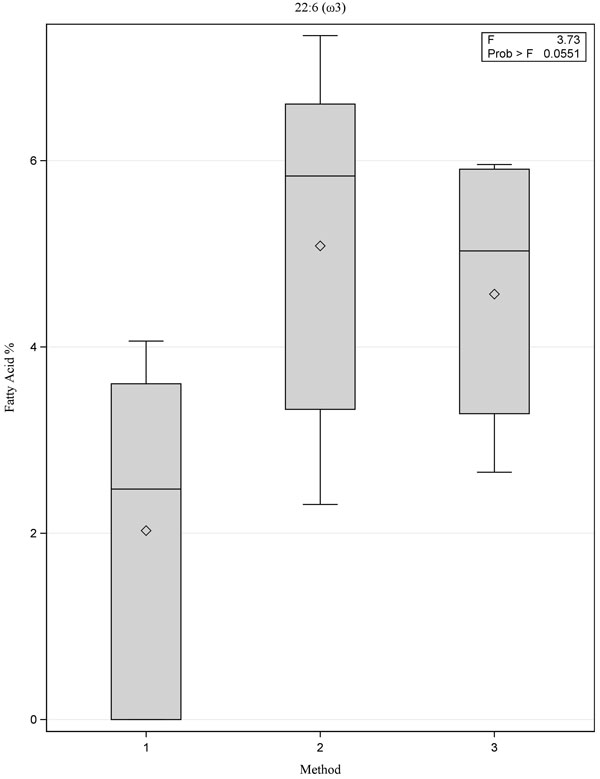 |
Fig. (3) Box plot showing comparison of fatty acid 22:6 (ω3) for the three methods, p=0.06. |
RESULTS AND DISCUSSION
The results show that there were no statistically significant differences between the three methods for the fatty acids, [16:0 (p=0.10), 18:0 (p=0.40), 18:1(ω9) (p=0.29), 18:2(ω6) (p=0.95), 18:3(ω3) (p=0.50), 20:5(ω3) (p=0.56), 22:6(ω3) (p=0.06)] and ω-3 index (p = 0.11) except for 20:4(ω6), (P=0.02). Our results are in agreement with previously reported data of total erythrocyte membrane fatty acid composition [12Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008; 47(5): 348-80.
[http://dx.doi.org/10.1016/j.plipres.2008.03.003] [PMID: 18435934] , 20Agren JJ, Törmälä ML, Nenonen MT, Hänninen OO. Fatty acid composition of erythrocyte, platelet, and serum lipids in strict vegans. Lipids 1995; 30(4): 365-9.
[http://dx.doi.org/10.1007/BF02536047] [PMID: 7609607] , 21Dougherty RM, Galli C, Ferro-Luzzi A, Iacono JM. Lipid and phospholipid fatty acid composition of plasma, red blood cells, and platelets and how they are affected by dietary lipids: a study of normal subjects from Italy, Finland, and the USA. Am J Clin Nutr 1987; 45(2): 443-55.
[PMID: 3812343] ]. Although there were no statistically significant differences between the three methods, Method 1 yielded lower values for the ω-3 fatty acids 20:5(ω3) and 22:6(ω3) when compared with Methods 2 and 3 (Figs. 1 -3
-3 ). We also observed wide variability between the measured values for fatty acids 20:5(ω3) and 22:6(ω3). This accounts for the low ω-3 index value reported for Method 1 because the omega-3 index value is calculated using these two fatty acids (Fig. 4
). We also observed wide variability between the measured values for fatty acids 20:5(ω3) and 22:6(ω3). This accounts for the low ω-3 index value reported for Method 1 because the omega-3 index value is calculated using these two fatty acids (Fig. 4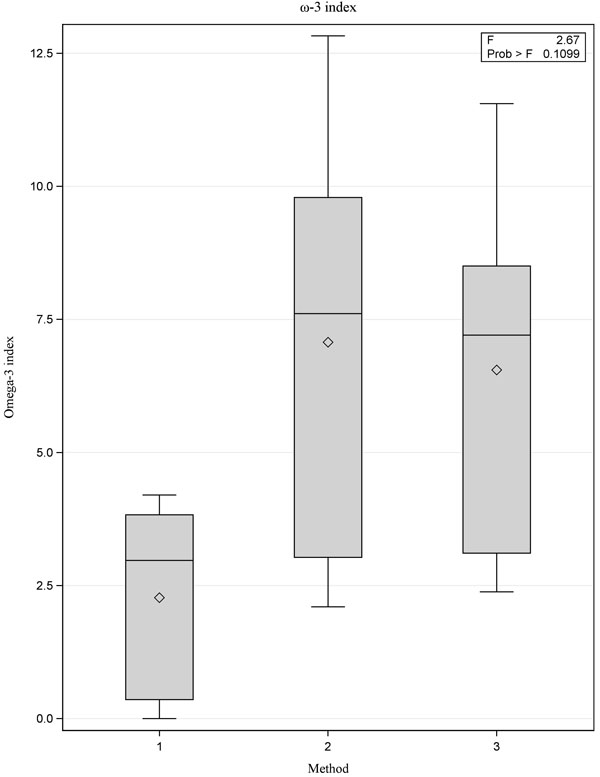 ).
).
 |
Fig. (4) Box plot showing comparison of ω-3 index values for the three methods, p=0.11. |
From the comparison of results obtained by measuring a representative set of red blood cell fatty acids and ω-3 index, we observed that there were no significant differences between the Hara and Radin [39Hara A, Radin NS. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 1978; 90(1): 420-6.
[http://dx.doi.org/10.1016/0003-2697(78)90046-5] [PMID: 727482] ], Harris et al. [10Harris WS, Pottala JV, Vasan RS, Larson MG, Robins SJ. Changes in erythrocyte membrane trans and marine fatty acids between 1999 and 2006 in older Americans. J Nutr 2012; 142(7): 1297-303.
[http://dx.doi.org/10.3945/jn.112.158295] [PMID: 22623386] ] and the sodium methoxide methods as shown in Table 2. The use of sodium methoxide for methylation of the red blood cells has not in any way altered the derivatization of fatty acid methyl esters as there was no difference in relative fatty acid composition between the three methods. Our results also agree with the findings of Kang and Wang [32Kang JX, Wang J. A simplified method for analysis of polyunsaturated fatty acids. BMC Biochem 2005; 6: 5.
[http://dx.doi.org/10.1186/1471-2091-6-5] [PMID: 15790399] ] that a prior extraction step is not necessary in fatty acid profiling of red blood cells and can be conveniently omitted to yield desirable outcomes.
Desirable aspects of Method 3 that makes it highly recommended and a better choice over Methods 1 and 2 are:
- It proceeds at room temperature and does not require heating.
- It is safer and can be used in very inexpensive open test tubes. Methods 1 and 2 require use of screw-capped tubes for the methylation step because 14% boron trifluoride is highly volatile and toxic if inhaled. Also the tubes can burst due to the pressure buildup.
- It is rapid with extraction and methylation in 10 minutes.
- It costs less because sodium methoxide has a longer shelf life when compared with 14% boron trifluoride-methanol (about 4 months and has to be refrigerated at all times) [40Teo CL, Idris A. Enhancing the various solvent extraction method via microwave irradiation for extraction of lipids from marine microalgae in biodiesel production. Bioresour Technol 2014; 171: 477-81.
[http://dx.doi.org/10.1016/j.biortech.2014.08.024] [PMID: 25201293] -42Fulk WK, Shorb MS. Production of an artifact during methanolysis of lipids by boron trifluoride-methanol. J Lipid Res 1970; 11(3): 276-7.
[PMID: 5441255] ] and sodium methoxide is much less expensive especially when prepared from sodium and methanol as described herein. - Sodium methoxide does not liberate aldehydes from plasmalogens as compared with the use of boron trifluoride in methanol [26Eder K. Gas chromatographic analysis of fatty acid methyl esters. J Chromatogr B Biomed Appl 1995; 671(1-2): 113-31.
[http://dx.doi.org/10.1016/0378-4347(95)00142-6] [PMID: 8520689] , 43Eder K, Reichlmayr-Lais AM, Kirchgessner M. Studies on the methanolysis of small amounts of purified phospholipids for gas chromatographic analysis of fatty acid methyl esters. J Chromatogr A 1992; 607(1): 55-67.
[http://dx.doi.org/10.1016/0021-9673(92)87054-C] [PMID: 1447360] ]. Hence, dimethyl acetals (DMAs) are not formed during esterification. This is especially important when working with Red Blood Cells [26Eder K. Gas chromatographic analysis of fatty acid methyl esters. J Chromatogr B Biomed Appl 1995; 671(1-2): 113-31.
[http://dx.doi.org/10.1016/0378-4347(95)00142-6] [PMID: 8520689] , 31Laryea MD, Cieslicki P, Diekmann E, Wendel U. Analysis of the fatty acid composition of erythrocyte phospholipids by a base catalysed transe sterification method prevention of formation of dimethylacetals. Clin Chim Acta 1988; 171(1): 11-8.
[http://dx.doi.org/10.1016/0009-8981(88)90286-0] [PMID: 3349633] , 43Eder K, Reichlmayr-Lais AM, Kirchgessner M. Studies on the methanolysis of small amounts of purified phospholipids for gas chromatographic analysis of fatty acid methyl esters. J Chromatogr A 1992; 607(1): 55-67.
[http://dx.doi.org/10.1016/0021-9673(92)87054-C] [PMID: 1447360] -45Lemaitre-Delaunay D, Pachiaudi C, Laville M, Pousin J, Armstrong M, Lagarde M. Blood compartmental metabolism of docosahexaenoic acid (DHA) in humans after ingestion of a single dose of [(13)C]DHA in phosphatidylcholine. J Lipid Res 1999; 40(10): 1867-74.
[PMID: 10508206] ]. - Using the sodium methoxide method should yield more consistent results in comparison with Method 1 as depicted by the high variability seen with results from Method 1. This could be attributed to the use of a single tube in the Method 2 and 3 experiments as opposed to Method 1 that requires two steps and the changing of tubes in between extraction and methylation phases. During the change of tubes, there may be partial loss of the samples, which can lead to incomplete recovery of FAMEs.
- Method 2 described by Harris [10Harris WS, Pottala JV, Vasan RS, Larson MG, Robins SJ. Changes in erythrocyte membrane trans and marine fatty acids between 1999 and 2006 in older Americans. J Nutr 2012; 142(7): 1297-303.
[http://dx.doi.org/10.3945/jn.112.158295] [PMID: 22623386] ] is very similar to the simplified method for analysis of polyunsaturated fatty acids which was described by Kang and Wang [32Kang JX, Wang J. A simplified method for analysis of polyunsaturated fatty acids. BMC Biochem 2005; 6: 5.
[http://dx.doi.org/10.1186/1471-2091-6-5] [PMID: 15790399] ]. However, the major difference in the protocol is the length of time required for the heating phase. Kang and Wang heated the samples at 100˚C for 1 hour while Harris et al. heated at the same temperature for 10 mins. The differences in samples analyzed might be accountable for the differences in the heating times. - Our results not only show that sodium methoxide is an effective methylating agent in RBC fatty acid analysis, they also further validate that a prior extraction step can be conveniently omitted to achieve the desired outcome. An overview of common reagents that have been used to analyze RBC fatty acids over the past 20 years is summarized in Table 3.
CONCLUSION
In conclusion, our results demonstrate that sodium methoxide can be used effectively in a single step extraction and methylation process for red blood cell fatty acid analysis. this was validated vs. the notable Hara and Radan [39Hara A, Radin NS. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 1978; 90(1): 420-6.
[http://dx.doi.org/10.1016/0003-2697(78)90046-5] [PMID: 727482] ] method and the Harris et al. method [10Harris WS, Pottala JV, Vasan RS, Larson MG, Robins SJ. Changes in erythrocyte membrane trans and marine fatty acids between 1999 and 2006 in older Americans. J Nutr 2012; 142(7): 1297-303.
[http://dx.doi.org/10.3945/jn.112.158295] [PMID: 22623386] ]. Furthermore, we have described in detail the steps involved in using sodium methoxide not previously reported. This simplified sodium methoxide method is less time consuming, safer, less expensive and yields results not significantly different from the well-known use of boron trifluoride-methanol. It allows for high throughput analysis of fatty acids including long chain fatty acids which are of utmost importance in calculating the omega-3 index in erythrocytes.
NOTES
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
This study was supported by the National Institute of Nursing Research, 5R01NR013430-03. We gratefully acknowledge the expert assistance and advice of Scott Serdoz, Hirotada Fukushige, Maythem AL-Amery and Will Serson. Special thanks to Sarah Janse of the Statistics Department, University of Kentucky for her immense help.
REFERENCES
| [1] | Liu RL, Zhang J, Mou ZL, Hao SL, Zhang ZQ. Microwave-assisted one-step extraction-derivatization for rapid analysis of fatty acids profile in herbal medicine by gas chromatography-mass spectrometry. Analyst (Lond) 2012; 137(21): 5135-43. [http://dx.doi.org/10.1039/c2an36178g] [PMID: 22968083] |
| [2] | Harris WS, Pottala JV, Varvel SA, Borowski JJ, Ward JN, McConnell JP. Erythrocyte omega-3 fatty acids increase and linoleic acid decreases with age: observations from 160,000 patients. Prostaglandins Leukot Essent Fatty Acids 2013; 88(4): 257-63. [http://dx.doi.org/10.1016/j.plefa.2012.12.004] [PMID: 23375840] |
| [3] | von Schacky C. Omega-3 Index and sudden cardiac death. Nutrients 2010; 2(3): 375-88. [http://dx.doi.org/10.3390/nu2030375] [PMID: 22254028] |
| [4] | Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr 2003; 77(2): 319-25. [PMID: 12540389] |
| [5] | Grindel A, Staps F, Kuhnt K. Cheek cell fatty acids reflect n-3 PUFA in blood fractions during linseed oil supplementation: a controlled human intervention study. Lipids Health Dis 2013; 12: 173. [http://dx.doi.org/10.1186/1476-511X-12-173] [PMID: 24229084] |
| [6] | Puca AA, Andrew P, Novelli V, et al. Fatty acid profile of erythrocyte membranes as possible biomarker of longevity. Rejuvenation Res 2008; 11(1): 63-72. [http://dx.doi.org/10.1089/rej.2007.0566] [PMID: 18160025] |
| [7] | Roy S, Brasky TM, Belury MA, et al. Associations of erythrocyte ω-3 fatty acids with biomarkers of ω-3 fatty acids and inflammation in breast tissue. Int J Cancer 2015; 137(12): 2934-46. [http://dx.doi.org/10.1002/ijc.29675] [PMID: 26137879] |
| [8] | Harris WS. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr 2008; 87(6): 1997S-2002S. [PMID: 18541601] |
| [9] | Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med 2004; 39(1): 212-20. [http://dx.doi.org/10.1016/j.ypmed.2004.02.030] [PMID: 15208005] |
| [10] | Harris WS, Pottala JV, Vasan RS, Larson MG, Robins SJ. Changes in erythrocyte membrane trans and marine fatty acids between 1999 and 2006 in older Americans. J Nutr 2012; 142(7): 1297-303. [http://dx.doi.org/10.3945/jn.112.158295] [PMID: 22623386] |
| [11] | Von Schacky C. Omega-3 fatty acids vs. cardiac diseasethe contribution of the omega-3 index. Cell Mol Biol (Noisy-le-grand) 2010; 56(1): 93-101. [PMID: 20196973] |
| [12] | Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008; 47(5): 348-80. [http://dx.doi.org/10.1016/j.plipres.2008.03.003] [PMID: 18435934] |
| [13] | Takigawa H, Nakagawa H, Kuzukawa M, Mori H, Imokawa G. Deficient production of hexadecenoic acid in the skin is associated in part with the vulnerability of atopic dermatitis patients to colonization by Staphylococcus aureus. Dermatology (Basel) 2005; 211(3): 240-8. [http://dx.doi.org/10.1159/000087018] [PMID: 16205069] |
| [14] | Klingler M, Koletzko B. Novel methodologies for assessing omega-3 fatty acid status - a systematic review. Br J Nutr 2012; 107(Suppl. 2): S53-63. [http://dx.doi.org/10.1017/S0007114512001468] [PMID: 22591903] |
| [15] | Al-Tamer YY, Mahmood AA. Fatty-acid composition of the colostrum and serum of fullterm and preterm delivering Iraqi mothers. Eur J Clin Nutr 2004; 58(8): 1119-24. [http://dx.doi.org/10.1038/sj.ejcn.1601939] [PMID: 15054424] |
| [16] | Klingler M, Klem S, Demmelmair H, Koletzko B. Comparison of the incorporation of orally administered DHA into plasma, erythrocyte and cheek cell glycerophospholipids. Br J Nutr 2013; 109(5): 962-8. [http://dx.doi.org/10.1017/S000711451200222X] [PMID: 22874641] |
| [17] | Clayton EH, Gulliver CE, Piltz JW, Taylor RD, Blake RJ, Meyer RG. Improved extraction of saturated fatty acids but not omega-3 fatty acids from sheep red blood cells using a one-step extraction procedure. Lipids 2012; 47(7): 719-27. [http://dx.doi.org/10.1007/s11745-012-3674-1] [PMID: 22570172] |
| [18] | Harris WS, Thomas RM. Biological variability of blood omega-3 biomarkers. Clin Biochem 2010; 43(3): 338-40. [http://dx.doi.org/10.1016/j.clinbiochem.2009.08.016] [PMID: 19733159] |
| [19] | Tepsic J, Vucic V, Arsic A, Blazencic-Mladenovic V, Mazic S, Glibetic M. Plasma and erythrocyte phospholipid fatty acid profile in professional basketball and football players. Eur J Appl Physiol 2009; 107(3): 359-65. [http://dx.doi.org/10.1007/s00421-009-1131-5] [PMID: 19633987] |
| [20] | Agren JJ, Törmälä ML, Nenonen MT, Hänninen OO. Fatty acid composition of erythrocyte, platelet, and serum lipids in strict vegans. Lipids 1995; 30(4): 365-9. [http://dx.doi.org/10.1007/BF02536047] [PMID: 7609607] |
| [21] | Dougherty RM, Galli C, Ferro-Luzzi A, Iacono JM. Lipid and phospholipid fatty acid composition of plasma, red blood cells, and platelets and how they are affected by dietary lipids: a study of normal subjects from Italy, Finland, and the USA. Am J Clin Nutr 1987; 45(2): 443-55. [PMID: 3812343] |
| [22] | Skeaff CM, Hodson L, McKenzie JE. Dietary-induced changes in fatty acid composition of human plasma, platelet, and erythrocyte lipids follow a similar time course. J Nutr 2006; 136(3): 565-9. [PMID: 16484525] |
| [23] | Innis SM, Kuhnlein HV, Kinloch D. The composition of red cell membrane phospholipids in Canadian Inuit consuming a diet high in marine mammals. Lipids 1988; 23(11): 1064-8. [http://dx.doi.org/10.1007/BF02535653] [PMID: 3237006] |
| [24] | Prisco D, Filippini M, Francalanci I, et al. Effect of n-3 polyunsaturated fatty acid intake on phospholipid fatty acid composition in plasma and erythrocytes. Am J Clin Nutr 1996; 63(6): 925-32. [PMID: 8644688] |
| [25] | Magnusardottir AR, Steingrimsdottir L, Thorgeirsdottir H, Gunnlaugsson G, Skuladottir GV. Docosahexaenoic acid in red blood cells of women of reproductive age is positively associated with oral contraceptive use and physical activity. Prostaglandins Leukot Essent Fatty Acids 2009; 80(1): 27-32. [http://dx.doi.org/10.1016/j.plefa.2008.10.004] [PMID: 19071003] |
| [26] | Eder K. Gas chromatographic analysis of fatty acid methyl esters. J Chromatogr B Biomed Appl 1995; 671(1-2): 113-31. [http://dx.doi.org/10.1016/0378-4347(95)00142-6] [PMID: 8520689] |
| [27] | Otsuka R, Kato Y, Imai T, Ando F, Shimokata H. Higher serum EPA or DHA, and lower ARA compositions with age independent fatty acid intake in Japanese aged 40 to 79. Lipids 2013; 48(7): 719-27. [http://dx.doi.org/10.1007/s11745-013-3763-9] [PMID: 23389403] |
| [28] | Shantha NC, Napolitano GE. Gas chromatography of fatty acids. J Chromatogr A 1992; 624(1-2): 37-51. [http://dx.doi.org/10.1016/0021-9673(92)85673-H] [PMID: 1494015] |
| [29] | Rodríguez-Palmero M, Lopez-Sabater MC, Castellote-Bargallo AI, De la Torre-Boronat MC, Rivero-Urgell M. Comparison of two methods for the determination of fatty acid profiles in plasma and erythrocytes. J Chromatogr A 1998; 793(2): 435-40. [http://dx.doi.org/10.1016/S0021-9673(97)10554-4] [PMID: 9474792] |
| [30] | Di Marino L, Maffettone A, Cipriano P, et al. Is the erythrocyte membrane fatty acid composition a valid index of skeletal muscle membrane fatty acid composition? Metabolism 2000; 49(9): 1164-6. [http://dx.doi.org/10.1053/meta.2000.8616] [PMID: 11016898] |
| [31] | Laryea MD, Cieslicki P, Diekmann E, Wendel U. Analysis of the fatty acid composition of erythrocyte phospholipids by a base catalysed transe sterification method prevention of formation of dimethylacetals. Clin Chim Acta 1988; 171(1): 11-8. [http://dx.doi.org/10.1016/0009-8981(88)90286-0] [PMID: 3349633] |
| [32] | Kang JX, Wang J. A simplified method for analysis of polyunsaturated fatty acids. BMC Biochem 2005; 6: 5. [http://dx.doi.org/10.1186/1471-2091-6-5] [PMID: 15790399] |
| [33] | Bell JG, Mackinlay EE, Dick JR, Younger I, Lands B, Gilhooly T. Using a fingertip whole blood sample for rapid fatty acid measurement: method validation and correlation with erythrocyte polar lipid compositions in UK subjects. Br J Nutr 2011; 106(9): 1408-15. [http://dx.doi.org/10.1017/S0007114511001978] [PMID: 21736805] |
| [34] | Coviello G, Tutino V, Notarnicola M, Caruso MG. Erythrocyte membrane fatty acids profile in colorectal cancer patients: a preliminary study. Anticancer Res 2014; 34(9): 4775-9. [PMID: 25202057] |
| [35] | Koehrer P, Saab S, Berdeaux O, et al. Erythrocyte phospholipid and polyunsaturated fatty acid composition in diabetic retinopathy. PLoS One 2014; 9(9): e106912. [http://dx.doi.org/10.1371/journal.pone.0106912] [PMID: 25188352] |
| [36] | Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 1986; 27(1): 114-20. [PMID: 3958609] |
| [37] | Lin YH, Hanson JA, Strandjord SE, et al. Fast transmethylation of total lipids in dried blood by microwave irradiation and its application to a population study. Lipids 2014; 49(8): 839-51. [http://dx.doi.org/10.1007/s11745-014-3918-3] [PMID: 24986160] |
| [38] | Segura R. Preparation of fatty acid methyl esters by direct transesterification of lipids with aluminium chloride-methanol. J Chromatogr A 1988; 441(1): 99-113. [http://dx.doi.org/10.1016/S0021-9673(01)84658-6] [PMID: 3403681] |
| [39] | Hara A, Radin NS. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 1978; 90(1): 420-6. [http://dx.doi.org/10.1016/0003-2697(78)90046-5] [PMID: 727482] |
| [40] | Teo CL, Idris A. Enhancing the various solvent extraction method via microwave irradiation for extraction of lipids from marine microalgae in biodiesel production. Bioresour Technol 2014; 171: 477-81. [http://dx.doi.org/10.1016/j.biortech.2014.08.024] [PMID: 25201293] |
| [41] | Böcking C, Nockher Wolfgang A, Schreiner M, Renz H, Pfefferle Petra I. Development and validation of a combined method for the biomonitoring of omega-3/-6 fatty acids and conjugated linoleic acids in different matrices from human and nutritional sources. Clin Chem Lab Med 2010; 48(12): 1757-63. |
| [42] | Fulk WK, Shorb MS. Production of an artifact during methanolysis of lipids by boron trifluoride-methanol. J Lipid Res 1970; 11(3): 276-7. [PMID: 5441255] |
| [43] | Eder K, Reichlmayr-Lais AM, Kirchgessner M. Studies on the methanolysis of small amounts of purified phospholipids for gas chromatographic analysis of fatty acid methyl esters. J Chromatogr A 1992; 607(1): 55-67. [http://dx.doi.org/10.1016/0021-9673(92)87054-C] [PMID: 1447360] |
| [44] | Araujo P, Nguyen TT, Frøyland L, Wang J, Kang JX. Evaluation of a rapid method for the quantitative analysis of fatty acids in various matrices. J Chromatogr A 2008; 1212(1-2): 106-13. [http://dx.doi.org/10.1016/j.chroma.2008.10.006] [PMID: 18937951] |
| [45] | Lemaitre-Delaunay D, Pachiaudi C, Laville M, Pousin J, Armstrong M, Lagarde M. Blood compartmental metabolism of docosahexaenoic acid (DHA) in humans after ingestion of a single dose of [(13)C]DHA in phosphatidylcholine. J Lipid Res 1999; 40(10): 1867-74. [PMID: 10508206] |