- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Nutrition Journal
(Discontinued)
ISSN: 1874-2882 ― Volume 15, 2021
Feed Intake Patterns and Immediate Glycaemic and Insulinaemic Responses of Horses Following Ingestion of Different Quantities of Starch From Oat, Barley and Grains
Annette Zeyner1, *, Kristin Romanowski2, Aileen Orgis2, Andreas Vernunft2, 3, Jutta Gottschalk4, Almuth Einspanier4, Gabor Koeller5, Monika Wensch-Dorendorf6
Abstract
Background:
Relevant literature indicate that more than 0.8 g starch/kg body weight from compounded feed composed of different starch sources induces disproportionate glycaemic and insulinaemic responses in horses.
Objective:
It should be investigated whether crushed oats, barley and maize also cause a disproportionate increase in plasma glucose and insulin when fed as the only concentrate in quantities equal to and above 0.8 g starch/kg body weight.
Method:
Four mares received hay plus oats, barley and maize, respectively, in quantities equal to 0.8, 1.0 and 2.0 g starch/kg body weight. At the test days, chewing parameters were detected and blood sampled before and 30, 60, 90 and 120 min after the concentrate meal. Plasma glucose and insulin were measured and areas under the curve were calculated.
Results:
Maize was ingested particularly slowly (dry matter basis; P < 0.05), but glycaemic and insulinaemic responses were particularly low (starch basis; P < 0.05). In general, the glycaemic responses were highest with 1 g starch/kg body weight (P < 0.05). The quantity of starch had no effect on the insulinaemic response (P > 0.05). A defined increase in plasma glucose induced the highest insulinaemic response with oat grains.
Conclusion:
Oats and barley are ingested faster and induce higher glycaemic and insulinaemic responses than maize. Until 120 min postprandial, elevated quantities of starch from these grains seem to induce no disproportionate or at least linear increase of plasma glucose and insulin. The insulinaemic response to a defined increase of plasma glucose is particularly pronounced with oats.
Article Information
Identifiers and Pagination:
Year: 2017Volume: 11
First Page: 39
Last Page: 51
Publisher Id: TONUTRJ-11-39
DOI: 10.2174/1874288201711010039
Article History:
Received Date: 03/03/2017Revision Received Date: 20/04/2017
Acceptance Date: 23/04/2017
Electronic publication date: 21/06/2017
Collection year: 2017
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Institute of Agricultural and Nutritional Science, Group Animal Nutrition, Martin Luther University Halle-Wittenberg, Theodor-Lieser-Str. 11, D-06120 Halle (Saale) Germany; Tel: ++49 (0)345 55-22716; fax: ++49 (0)345 55-27050; E-mail: annette.zeyner@landw.uni-halle.de
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 03-03-2017 |
Original Manuscript | Feed Intake Patterns and Immediate Glycaemic and Insulinaemic Responses of Horses Following Ingestion of Different Quantities of Starch From Oat, Barley and Grains | |
INTRODUCTION
Starch from cereal grains is the most important energy source for performance horses and in some cases also for other equines which shell get starchy concentrates for various reasons [1Zeyner A. Energy providing nutrient sources. In: Saastamoinen MT, Martin-Rosset W, Eds. Nutrition of the exercising horse EAAP publication no 125. Wageningen, The Netherlands: Wageningen Academic Publishers 2008; pp. 277-94.]. Oat, barley and maize grains are the most used cereal grains in horse nutrition. They are prominent components of compound feeds. In this case, barley and maize are often processed. Nevertheless, cereal grains might also be fed in isolation or in combination with a mineral mix and being usually crushed or cracked.
Starch from cereal grains is provided to be decomposed by endogenous amylase in the small intestine of the horse. Glucose as the cleaving product can then be absorbed inducing an insulinaemic response as immediate metabolic response. In a species comparison, however, the activity of endogenous amylase is particularly low in horses [2Radicke S, Landes E, Kienzle E, Meyer H. Aktivität der Amylase im Darmkanal des Pferdes in Abhängigkeit von der Futterart Pferdeheilkunde. Sonderausgabe 1992; pp. 99-102.]. Thus, a safe limit of starch supply is requested to ensure effective absorption of glucose, and furthermore to avoid starch overload of the caecum with accompanying excessive microbial fermentation and, in the worst case, caecal acidosis [3Garner HE, Coffman JR, Hahn AW, Hutcheson DP, Tumbleson ME. Equine laminitis of alimentary origin: an experimental model. Am J Vet Sci 1975; 30: 441-4.]. Studies addressing the digestibility of starch until the end of the small intestine in horses confirm the necessity of such a limit [for overview see 1Zeyner A. Energy providing nutrient sources. In: Saastamoinen MT, Martin-Rosset W, Eds. Nutrition of the exercising horse EAAP publication no 125. Wageningen, The Netherlands: Wageningen Academic Publishers 2008; pp. 277-94., 4Meyer H, Radicke S, Kienzle E, Wilke S, Kleffken D, Illenseer M. Investigations on preileal digestion of starch from grain, potato and manioc in horses. J Vet Med A 1995; 42: 371-81.
[http://dx.doi.org/10.1111/j.1439-0442.1995.tb00389.x] , 5Julliand V, De Frombelle A, Varloud M. Starch digestion in horses: The impact of feed processing. Livest Sci 2006; 100: 44-52.
[http://dx.doi.org/10.1016/j.livprodsci.2005.11.001] , 6Kienzle E, Pohlenz J, Radicke S. Morphology of starch digestion in the horse. J Vet Med A 1997; 44: 207-21.
[http://dx.doi.org/10.1111/j.1439-0442.1997.tb01103.x] ]. From this, it is suggested that a starch supply until at most 2 g/kg body weight (BW) and meal ensures a predominant digestion before the digesta passes into the hindgut. In this concern, the type of cereal grain and thermal processing are main varying factors [4Meyer H, Radicke S, Kienzle E, Wilke S, Kleffken D, Illenseer M. Investigations on preileal digestion of starch from grain, potato and manioc in horses. J Vet Med A 1995; 42: 371-81.
[http://dx.doi.org/10.1111/j.1439-0442.1995.tb00389.x] , 5Julliand V, De Frombelle A, Varloud M. Starch digestion in horses: The impact of feed processing. Livest Sci 2006; 100: 44-52.
[http://dx.doi.org/10.1016/j.livprodsci.2005.11.001] ]. While feeding as either native ore broken grain seems to make not a main difference [4Meyer H, Radicke S, Kienzle E, Wilke S, Kleffken D, Illenseer M. Investigations on preileal digestion of starch from grain, potato and manioc in horses. J Vet Med A 1995; 42: 371-81.
[http://dx.doi.org/10.1111/j.1439-0442.1995.tb00389.x] , 5Julliand V, De Frombelle A, Varloud M. Starch digestion in horses: The impact of feed processing. Livest Sci 2006; 100: 44-52.
[http://dx.doi.org/10.1016/j.livprodsci.2005.11.001] ], there is an obvious substantial distinction between individual grain types. Starch from oat grains was in every case highly digestible until the end of the small intestine, even if provided in high quantities. On the contrary, starch from barley and maize grains seems to be rather low or even very low praecacal digestible. A main reason for this might be differences in the morphology of the starch granules from the individual types of cereal grains, meaning their size, the strength of the connection among each other, and their embedding into accompanying structures [6Kienzle E, Pohlenz J, Radicke S. Morphology of starch digestion in the horse. J Vet Med A 1997; 44: 207-21.
[http://dx.doi.org/10.1111/j.1439-0442.1997.tb01103.x] , 7Bochnia M, Walther S, Schenkel H, Romanowski K, Zeyner A. Comparison of scanning electron microscopic examination of oats, barley and maize grains with the analyzed degree of starch breakdown and glycaemic responses in horses. IJSRSET 2015; 1(2): 81-4.].
Measuring the precaecal digestibility is the only way to quantify the amount of starch entering the hindgut under defined feeding conditions. Conversely, it is not possible to conclude that the precaecal digestible part of starch was completely broken down to glucose and absorbed in the small intestine. This is why starch such as other easily available carbohydrates started to be fermented by microbes already in the lower gut, particularly in the Pars nonglandularis of the horses’ stomach. Despite the quantity of starch that is subject of this fate can yet not be predicted, evidence exists that organic acids as end products of microbial fermentation elevate the risk for gastric ulcers in horses disproportionately when more than 1 g starch per kg of BW and meal is fed [8Luthersson N, Hou Nielsen K, Harris P, Parkin TD. Risk factors associated with equine gastric ulceration syndrome (EGUS) in 201 horses in Denmark. Equine Vet J 2009; 41: 625-30.
[http://dx.doi.org/10.2746/042516409X441929] ].
Concerning that microbial fermentation already starts in the lower gut, it makes sense to consider the detection of postprandial (ppr.) glycaemic and insulinaemic responses as valuable method to assess the contribution of any starch source to the metabolic glucose supply of dietary origin. Furthermore, this method is suitable to judge the ppr. dynamics of plasma glucose and insulin to avoid overwhelming responses which might be critical for horses with regard to their insulin sensitivity [9Treiber KH, Boston RC, Kronfeld DS, Staniar WB, Harris PA. Insulin resistance and compensation in Thoroughbred weanlings adapted to high glycemic meals. J Anim Sci 2005; 83: 2357-64.
[http://dx.doi.org/10.2527/2005.83102357x] ]. Respective studies have been performed to investigate the impact of processing oats, barley and maize grains on the glycaemic and insulinaemic responses when fed a defined quantity of starch [10Vervuert I, Coenen M, Bothe C. Effects of oat processing on the glycaemic and insulin responses in horses. J Anim Physiol Anim Nutr (Berl) 2003; 87: 96-104.
[http://dx.doi.org/10.1046/j.1439-0396.2003.00420.x] -12Vervuert I, Coenen M, Bothe C. Glycaemic and insulinaemic responses to mechanical or thermal processed barley in horses. J Anim Physiol Anim Nutr (Berl) 2007; 91: 263-8.
[http://dx.doi.org/10.1111/j.1439-0396.2007.00703.x] ]. The impact of increasing quantities of starch provided per meal has been studied with 0.3., 0.6, 0.8, 1.1, 1.4 and 2 g starch per kg of BW from compound feed composed of manifold starch sources [13Vervuert I, Voigt K, Hollands T, Cuddeford D, Coenen M. Effect of feeding increasing quantities of starch on glycaemic and insulinaemic responses in healthy horses. Vet J 2009; 182(1): 67-72.
[http://dx.doi.org/10.1016/j.tvjl.2008.04.011] ]. This study indicated that a starch supply above 0.8 g/kg BW and meal induces a disproportionate glycaemic and insulinaemic response at least when the starch sources are highly available.
To the authors’ knowledge, a direct comparison of the impact of oat, barley and maize grains fed to provide different amounts of starch on the glycaemic and insulinaemic response of adult healthy horses has not yet been performed. The aim of the recent study was to investigate the respective immediate metabolic reaction when crushed oat and barley grains and cracked maize grains are provided each one in quantities equal to 0.8, 1 and 2 g starch/kg BW and meal. In this concern, 0.8 g starch/kg of BW and meal should reflect the metabolic secure dose outlined by [13Vervuert I, Voigt K, Hollands T, Cuddeford D, Coenen M. Effect of feeding increasing quantities of starch on glycaemic and insulinaemic responses in healthy horses. Vet J 2009; 182(1): 67-72.
[http://dx.doi.org/10.1016/j.tvjl.2008.04.011] ]. 1 and 2 g starch/kg of BW, however, increasing quantities, which may lead to disproportionate glycaemic and insulinaemic responses. Furthermore, 1 g starch/kg of BW is the upper limit which is recommended not to exceed to avoid gastric ulcers [8Luthersson N, Hou Nielsen K, Harris P, Parkin TD. Risk factors associated with equine gastric ulceration syndrome (EGUS) in 201 horses in Denmark. Equine Vet J 2009; 41: 625-30.
[http://dx.doi.org/10.2746/042516409X441929] ] and therefore taken over as recommendation for feeding horses by the German respective key book [14Gesellschaft für Ernährungsphysiologie, Ed Energie- und Nährstoffbedarf landwirtschaftlicher Nutztiere 11, Empfehlungen zur Energie- und Nährstoffversorgung von Pferden. Frankfurt am Main: DLG-Verlag 2014.], and 2 g starch/kg BW in turn to obviate caecal starch overload [1Zeyner A. Energy providing nutrient sources. In: Saastamoinen MT, Martin-Rosset W, Eds. Nutrition of the exercising horse EAAP publication no 125. Wageningen, The Netherlands: Wageningen Academic Publishers 2008; pp. 277-94., 4Meyer H, Radicke S, Kienzle E, Wilke S, Kleffken D, Illenseer M. Investigations on preileal digestion of starch from grain, potato and manioc in horses. J Vet Med A 1995; 42: 371-81.
[http://dx.doi.org/10.1111/j.1439-0442.1995.tb00389.x] -6Kienzle E, Pohlenz J, Radicke S. Morphology of starch digestion in the horse. J Vet Med A 1997; 44: 207-21.
[http://dx.doi.org/10.1111/j.1439-0442.1997.tb01103.x] ]. Because an impact of the speed and intensity of starch intake cannot be excluded, feed intake and chewing activity accompanying should be monitored. The hypotheses were that, i) the highly available starch from oat grains induces a higher glycaemic and insulinaemic response than barley and maize, and ii) a starch supply above 0.8 g/kg of BW and meal causes disproportionate reactions in this concern.
MATERIALS AND METHOD
Animals and keeping of the animals: Four Warmblood-type mares (age 6.0 ± 1.6 years) with a body weight (BW) of 488 ± 29.7 kg (scale: AWE460, PAARI Waagen und Anlagenbau GmbH und Co. KG, Erfurt-Stotternheim, Germany) and a body condition score [15Kienzle E, Schramme S. Beurteilung des Ernährungszustandes mittels Body Condition Scores und Gewichtsschätzung beim adulten Warmblutpferd. Pferdeheilkunde 2004; 20(6): 517-24.
[http://dx.doi.org/10.21836/PEM20040604] ] of 5.2 ± 0.22/9 were used for the study. The mares were regularly dewormed and vaccinated and had a healthy dentition. During the experimental period, the horses were kept in individual boxes on rubber-mats and wood-shavings as bedding and had ad libitum access to tap water and a paddock without any edible material. On the measuring days, the horses had no turn out to the paddock.
Experimental design: Oat, barley and maize grains were fed in different quantities according to their starch content in which from each grain type 0.8, 1 and 2 g of starch/kg BW and meal, respectively, were provided during the individual feeding periods of the study. For this, a superordinate cross over-design was designed in which the three types of cereal grains were fed to the four horses. In this, a further cross over-design was imbedded where the individual starch quantities were fed within the individual grain types. At the end of each feeding events (3 types of cereal grains x 3 amounts of starch) during three consecutive test days each, chewing patterns were measured and blood sampled from the individual horses following provision of the morning meal. The entire feeding period lasted 8 weeks.
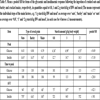
Treatments and diet composition: The horses received 1.5 kg of meadow hay per kg of BW in two equal meals per day. The hay was supplemented by cereal grains, which were different in their type and quantity within the individual feeding periods. For this, either crushed oats (variety ‘Melody’), crushed barley (variety ‘ACK 2927’) or cracked maize (variety ‘P9494’) were provided in quantities equal to 0.8, 1 or 2 g starch per kg of bwt and day. The analyzed chemical composition of all feedstuffs used in the study is given in (Table 1). According to the individual horses’ bwt, the addressed starch supply and the starch content of the cereal grain in question, the total quantity of cereal grains provided per meal varied as follows: 0.83 – 2.36 kg oats, 0.67 – 1.91 kg barley and 0.54 – 1.52 kg maize.
DM, dry matter; CA, crude ash; CP, crude protein; AEE, acid ether extract; CF, crude fiber; NDF, neutral detergent fiber; ADF, acid detergent fiber; ADL, acid detergent lignin; Hay 1, meadow hay used in the oats diet; Hay 2, meadow hay used in the barley and maize diets
Experimental period: On the test days, 1 kg of hay was given with the morning meal and 60 min thereafter the concentrate. During the concentrate intake, feed intake patterns including the chewing activity were measured and blood sampled for later analysis of plasma glucose and insulin.
Feed intake patterns were detected with the electronic device CARA, operating with piezoelectric effects [17Zeyner A, Quellmalz S, Dill J, Lengwenat O. Reproducibility of data on the chewing activity of horses measured by CARA. Proceedings of the 13th Congress of the European Society of Veterinary and Comparative Nutrition (ESVCN). 2009 Oct 15-17; 116.]. The measurement module was attached to a girth and combined with an individually adjustable chewing-halter, which was able to record the movement of maxilla and mandible through voltage change. Homogenous jaw movements were computed in sinus curves and one complete sinus curve interpreted as chewing cycle (CC). Any interruption of the succession of sinusoids was as break. Measured items and from these calculated parameters including breaks were called ‘total’, after mathematic removing of the breaks as ‘real’. To perform the measurement, the halter and girth were fixed to each horse prior to the presentation of the individual test meal. Recording of digital data started and stopped simultaneously to the consumption of the concentrates. The data were transmitted wireless to the CARA-innate analytical software.
Blood was sampled directly before (0 min) and during 30, 60, 90 and 120 min after the cereal grains were provided. For this, the Vena jugularis externa was fitted with an indwelling venous catheter (Braunüle MT Luer Look, REF 4206274, B. Braun Melsungen AG, Melsungen, Germany). The blood was collected in S-Monovettes (1.2 mg EDTA/ml; 1 mg Fluorid/ml, 2.7 ml FE; REF 05.1073, Sarstedt, Nümbrecht, Germany) for plasma glucose and in EDTA Monovettes (1.6 mg EDTA/9 ml K3E; REF 02.267.001, Sarstedt, Nümbrecht, Germany) for plasma insulin. The samples were stored at 4 °C until immediate centrifugation at 3.800 g for 10 min (centrifuge MLW T54; medicine technology Leipzig, Germany). Blood plasma was separated for subsequent analysis of glucose and insulin, transferred into Eppendorf vessels (Safe Seal micro tube; REF 72.695.500, Sarstedt, Nümbrecht, Germany) and stored at – 20 °C.
Chemical analyses: Feedstuffs and samples of blood plasma were undertaken chemical analyses.
From all feedstuffs, collective samples were taken during the study. The feed samples were ground to pass through a 1 mm sieve in a standard laboratory mill. For subsequent starch analysis, the oat grains were further milled in a ball mill. Afterwards, the content of dry matter (DM) and proximate nutrients were determined according to official methods of the Association of German Agricultural Research Laboratories ([16Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten, Ed Die chemische Untersuchung von Futtermitteln Loseblattsammlung mit Ergänzungslieferungen. Darmstadt: VDLUFA-Verlag 1976; III.Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch)]: methods no. 3.1: DM, 4.1.1: crude protein, 5.1.1B: acid ether extract, 6.1.1 crude fiber, 6.5.1 neutral detergent fiber, 6.5.2: acid detergent fiber, 6.5.3 acid detergent lignin 7.1.2: sugar and 8.1: crude ash). The starch content of the cereal grains was determined enzymatically referring to the amyloglucosidase method (for detail see [18Zeyner A, Gefrom A, Hillegeist D, Sommer M, Greef JM. Contribution to the method of sugar analysis in legume grains for ensiling – a pilot study. IJSRSET 2015; 1(2): 74-80.]). The analyzed chemical composition of the feedstuffs used in the study is given in (Table 1).
In the blood plasma, glucose was detected by the automated analyzer Hitachi 912 (Roche Diagnostics GmbH, Mannheim, Germany) as described by [19Köller G, Gieseler T, Schusser GF. Hämatologische und blutchemische Referenzbereiche bei Pferden unterschiedlicher Rasse und Altersgruppen basierend auf neuesten labordiagnostischen Methoden. Pferdeheilkunde 2014; 30(4): 381-93.]. Insulin was analyzed by a radioimmunoassay kit ([20Gottschalk J, Einspanier A, Ungemach FR, Abraham G. Influence of topical dexamethasone applications on insulin, glucose, thyroid hormone and cortisol levels in dogs. Res Vet Sci 2011; 90: 491-7.
[http://dx.doi.org/10.1016/j.rvsc.2010.06.026] ]: Insulin-Coa-ACount-RIA-Kit, BioSource Europe S.A., Belgium). From the results, the ppr. peak of plasma glucose and insulin and the respective time to reach these peaks within 120 min after provision of the starchy meal were identified. From the glycaemic and insulinaemic responses during this ppr. rising phase (until 120 min ppr.), the areas under the plasma glucose and insulin curve (AUCglu and AUCins), respectively, were determined by using a sum method of non-overlapping tri- and rectangles with the x axis and the zero value of y axis as baseline.
Feed intake patterns and calculations: From the measured feed intake patterns, the following parameters were calculated: i) total and real chewing frequency (in CC per sec), ii) chewing intensity (in CC/ kg DM), iii) duration of feed intake (in min/kg DM) differentiated into entire time for chewing plus breaks (feed intake time), chewing (chewing time) and breaks (break time) only, iv) total and real feed intake frequency (in g DM/min), v) intensity of starch intake (in g starch/CC), vi) frequency of starch intake (in g starch/sec).
Statistical analysis: Analysis was performed using the software package SPSS (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, versions 20 and 21, Armonk, NY: IBM Corp.). BW and body condition score of the horses at the beginning and the end of the study were compared by Wilcoxon test. Chewing patterns and blood parameters were evaluated basically as three-way ANOVA using the type of cereal grain (α), the amount of starch (β) and horse (γ) as fixed factors including all interactions:
yijkl=µ+αi+βj+γk+ (αβ)ij+ (αγ)ik+ (βγ)jk+ (αβγ)ijk+ eijkl
The model has been completed by taking into account the observations on three consecutive days as repeated observations within the SPSS software. Prior to this, the homogeneity of variance was tested by the Levene test. For post hoc comparison of means following analysis of variance, the Students-Newman-Keuls test was supplied. The differences of the concentrations of plasma glucose and insulin between the individual cereal grains and amounts of starch, respectively, at the different time points ppr. were compared by use of the Wilcoxon test. Furthermore, type and strength of the relationship between the ppr. glycaemic and insulinaemic responses were investigated by regression analysis. The level of statistical significance was pre-set at P < 0.05.
RESULTS
The horses’ remained healthy during the study and their BW (491 ± 32.4) and body condition score (5.2 ± 0.08) at the end of the experiment were not different from that at the beginning (P > 0.05).
The crushed grains from oats and barley had excellent acceptability. The cracked maize however was ingested more hesitant. Sometimes, the intake of the cracked maize grains was accompanied by a kind of licking behavior. This involved not only the intake of very fine particles from the through by licking, but also a licking on the horses’ own teeth. The average time of consumption of the starchy meals were 11 – 17 min/kg of DM with oats and barley at the bottom and maize at the upper end of the span. All evaluated patterns of feed intake and chewing activity were affected by the type of cereal grain (P < 0.001), but not by the quantity of starch and thus the amount of DM provided per meal (P > 0.05; Table 2). Furthermore, the individual horse had an impact on the time needed to ingest and chew the cereal grains (P < 0.05).
FIT, feed intake time (in min feed intake/kg DM with feed intake being the sum of CT and BT); CT, chewing time (in min chewing/kg DM); DM, dry matter; BT, break time (in min breaks/kg DM); CF, chewing frequency (in chewing cycles/sec); real, without breaks; total, with breaks; CI, chewing intensity (in CC/kg DM); CC, chewing cycles; FIF, feed intake frequency (in g DM/min)
In general, equal quantities of starch and DM, respectively, from cracked maize were ingested more slowly, and seemed to be chewed more intense than crushed oat and barley grains (P < 0.05; Table 3).
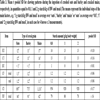
Mean ± pooled SD for chewing patterns during the ingestion of crushed oats and barley and cracked maize, respectively, in quantities equal to 0.8, 1 and 2 g starch/kg of BW and meal (The means represent the individual steps of the main factors, e.g. ‘1 g starch/kg BW and meal’ as average over ‘oats’, ‘barley’ and ‘maize’ or ‘oats’ as average over ‘0.8’, ‘1’ and ‘2 g starch/kg BW and meal’, in each case for 4 horses x 3 measurements).
FIT, feed intake time (in min feed intake/kg DM with feed intake being the sum of CT and BT); CT, chewing time (in min chewing/kg DM); DM, dry matter; BT, break time (in min breaks/kg DM); CF, chewing frequency (in chewing cycles/sec); real, without breaks; total, with breaks; CI, chewing intensity (in CC/kg DM); CC, chewing cycles; FIF, feed intake frequency (in g DM/min); a, b, c unequal superscripts indicate with P < 0.05 different means
In detail, the horses performed 26% and 46% less CC and needed 41% and 55% less time per kg of DM to eat oats and barley, respectively, compared to maize. The chewing frequency however was clearly higher with oats and barley than maize (P < 0.05). The characteristics of feed intake and chewing were very similar with oats and barley (P > 0.05), except for the chewing intensity where the horses invested 16% more CC per kg of DM to ingest oats vs barley (P < 0.05).
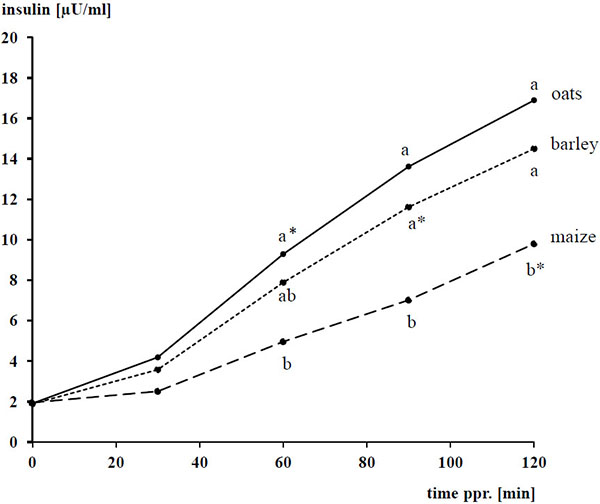
The basal concentrations of plasma glucose and insulin amounted to 4.93 ± 0.298 mmol/l and 1.8 ± 0.89 µU/ml, respectively, and were not affected by the diet (P > 0.05). After concentrate intake, plasma glucose and insulin increased following a convex curve Figs. (1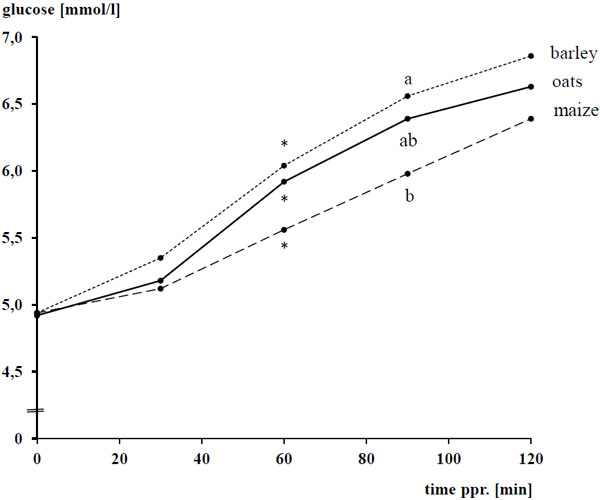 -4
-4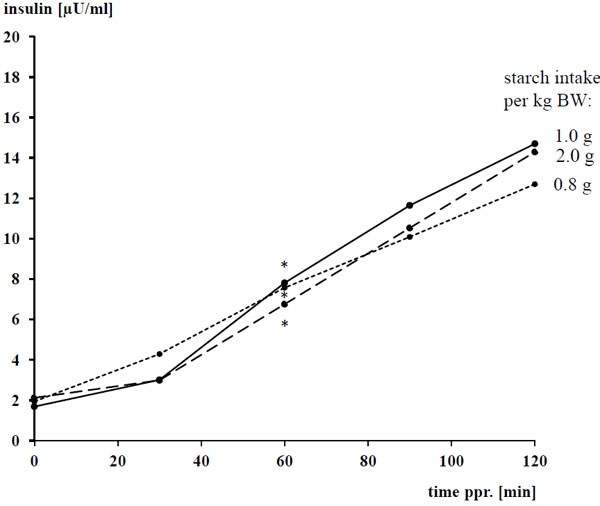 ) for both biochemical parameters, the difference to the respective basal level was significant between 30 - 120 min after concentrate feeding with a clear overcoming of the 60 min ppr.. The ppr. glucose peak was not influenced by the type of grain (P > 0.05), but highest with 1 g starch/kg BW compared 0.8 and 2 g starch/kg of BW, respectively (P < 0.05; Tables 4 and 5; Fig. (2
) for both biochemical parameters, the difference to the respective basal level was significant between 30 - 120 min after concentrate feeding with a clear overcoming of the 60 min ppr.. The ppr. glucose peak was not influenced by the type of grain (P > 0.05), but highest with 1 g starch/kg BW compared 0.8 and 2 g starch/kg of BW, respectively (P < 0.05; Tables 4 and 5; Fig. (2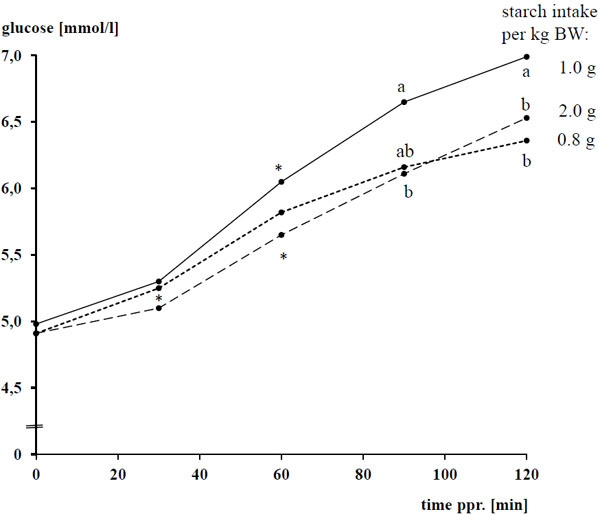 )).
)).
Peak, maximal postprandial (ppr.) concentration of plasma glucose (in mmol/l) or serum insulin (in µU/ml); Time to peak, time needed to reach the maximal ppr. concentration of glucose or insulin (in min ppr.); AUC, area under the 120 min ppr. plasma glucose (in mmol*min/l) or insulin (in µU*min/ml) curve over the respective basal levels
Regarding the ppr. insulin peak, there was an impact (P < 0.05) of the grain type but not the amount of starch (P > 0.05) with an interaction between both (P < 0.05). The ppr. insulin concentration reached 17 and 15 vs 19 µU/ml with oats and barley vs maize, respectively (P < 0.05). Including all measured items (n = 360), there was a strong linear dependency of ppr. plasma insulin (y, in µU/ml) from plasma glucose (x, in mmol/l) being described by the following equation: y = - 27.1 + 5.97 x (r = 0.804; p < 0.001). The relationship was strong linear until a glucose concentrations of ≈ 6 mmol/l and also between 7.5 – 8.5 mmol/l, the latter characterizes the upper border of the range. Between plasma glucose concentrations of ≈ 6 – 7.5 mmol/l a remarkable percentage of the measured insulin levels lied clearly above the linear regression line. Thus, the following exponential regression fits somewhat better than the described linear one: y = 0.022 exp0.936 x (r = 0.824; p < 0.001).
The type of cereal grain as well as the amount of starch affected the AUCglu (P < 0.05) while the AUCins was dependent from the type of cereal grain (P < 0.05), but not the amount of starch fed (P > 0.05). AUCglu and AUCins were lower with maize than oats and barley (P < 0.05). Similar to the ppr. glucose peak, the AUCglu was highest with 1 g starch/kg of BW (P < 0.05).
Mean ± pooled SD for items of the glycaemic and insulinaemic response following the ingestion of crushed oats and barley and cracked maize, respectively, in quantities equal to 0.8, 1 and 2 g starch/kg of BW and meal (The means represent the individual steps of the main factors, e.g. ‘1 g starch/kg BW and meal’ as average over ‘oats’, ‘barley’ and ‘maize’ or ‘oats’ as average over ‘0.8’, ‘1’ and ‘2 g starch/kg BW and meal’, in each case for 4 horses x 3 measurements).
Peak, maximal postprandial (ppr.) concentration of plasma glucose (in mmol/l) or serum insulin (in µU/ml); Time to peak, time needed to reach the maximal ppr. concentration of glucose or insulin (in min ppr.); AUC, area under the 120 min ppr. plasma glucose (in mmol*min/l) or insulin (in µU*min/ml) curve over the respective basal levels; a, b unequal superscripts indicate with P < 0.05 different means within ‘Type of cereal grain’ and ‘Starch amount’, respectively
DISCUSSION
The crushed oat grains were accepted very good which fits well with the anecdotal preference of horses for this type of cereal grain. While the barley was also eaten without any problems, the maize was ingested more hesitant which might in part had to do with the structures released by the crushing process being a mix of both course and very fine particles where the latter needs to be licked up. Furthermore, the mixture of crushed and chewed maize grains and saliva occasionally seemed to adhere to the teeth, which cost the horses additional efforts during the feed intake. It cannot be excluded, however, that such behavior may misinterpreted as chewing which would lead to false conclusions regarding the chewing intensity.
According to the individual horses’ BW and the starch content of the cereal grain in question, the total quantity of grains provided per meal varied widely. Within one type of cereal grains, the highest meal size provided to the same horse reached nearly the triple of the lowest. Despite of this high variation, the difference did not remarkably affect feed intake behavior and chewing activity, respectively. Taken both the time the horses needed to consume the concentrate meal and the starch content of the cereal grains into account, the highest quantity of starch per CC, respectively, was ingested from barley (0.842 and 1.025 g), followed by maize (0.744 and 0.876 g) and with some distance oats (0.581 and 0.750 g). The variability in meal size and the rate of feed consumption might have been responsible for differences in the digesta passage rate through the lower gut [21McLean BM, Hyslopp JJ, Longland AC, Cuddeford D, Hollands T. Physical processing of barley and its effects on intra-caecal fermentation patterns in ponies. Anim Feed Sci Technol 2000; 85: 79-87.
[http://dx.doi.org/10.1016/S0377-8401(00)00132-2] ] and thus together with the starch content of the cereal grain in question for the time course of glucose provision for absorption. On the other hand, evidence exists that the meal size might not substantially affect glycaemic responses in horses [22van Weyenberg S, Buyse J, Janssens GP. Digestibility of a complete ration in horses fed once ore three times a day and correlation with key blood parameters. Vet J 2007; 173: 311-6.
[http://dx.doi.org/10.1016/j.tvjl.2005.08.011] ].
In the recent study, the basal concentrations of plasma glucose and insulin as well as the ppr. responses in terms of absolute levels and synchronism did not indicate any metabolic disorder in this concern, despite more sophisticated tests would be necessary to be definitively conclusive [19Köller G, Gieseler T, Schusser GF. Hämatologische und blutchemische Referenzbereiche bei Pferden unterschiedlicher Rasse und Altersgruppen basierend auf neuesten labordiagnostischen Methoden. Pferdeheilkunde 2014; 30(4): 381-93., 23Kronfeld DS, Treiber KH, Hess TM, Boston RC. Insulin resistance in the horse: definition, detection, and dietetics. J Anim Sci 2005; 83: 1-10., 24Dunbar LK, Mielnicki KA, Dembek KA, Toribio RE, Burns TA. Evaluation of four diagnostic tests for insulin dysregulation in adult light-breed horses. J Vet Intern Med 2016; 30: 885-91.
[http://dx.doi.org/10.1111/jvim.13934] ]. Also the ppr. dynamics of both plasma glucose and insulin (regarding time to the first significant difference compared to the basal level, peak and time to peak) lie within the range of what is known from the literature following ingestion of similar amounts of starch from whole, crushed or ground cereal grains by adult healthy horses [10Vervuert I, Coenen M, Bothe C. Effects of oat processing on the glycaemic and insulin responses in horses. J Anim Physiol Anim Nutr (Berl) 2003; 87: 96-104.
[http://dx.doi.org/10.1046/j.1439-0396.2003.00420.x] -12Vervuert I, Coenen M, Bothe C. Glycaemic and insulinaemic responses to mechanical or thermal processed barley in horses. J Anim Physiol Anim Nutr (Berl) 2007; 91: 263-8.
[http://dx.doi.org/10.1111/j.1439-0396.2007.00703.x] , 25Zeyner A, Hoffmeister C, Einspanier A, Gottschalk J, Lengwenat O, Illies M. Glycaemic and insulinaemic response of Quarter Horses to concentrates high in fat and low in soluble carbohydrates. Equine Vet J Suppl 2006; 36: 643-7.
[http://dx.doi.org/10.1111/j.2042-3306.2006.tb05619.x] ].
In the recent study, barley induced the highest AUCglu followed, with a negligible distance, by oats and with a large distance by maize. The results for oats and maize come to the expectations because of the known high and low, respectively, availability of starch from native and broken grains of these cereal types [4Meyer H, Radicke S, Kienzle E, Wilke S, Kleffken D, Illenseer M. Investigations on preileal digestion of starch from grain, potato and manioc in horses. J Vet Med A 1995; 42: 371-81.
[http://dx.doi.org/10.1111/j.1439-0442.1995.tb00389.x] -6Kienzle E, Pohlenz J, Radicke S. Morphology of starch digestion in the horse. J Vet Med A 1997; 44: 207-21.
[http://dx.doi.org/10.1111/j.1439-0442.1997.tb01103.x] ]. The reason why barley was at least equal to oats with regard to the glycaemic response in the ppr. rising phase cannot be explained with retraction to the literature.
Particularly for crushed barley grains a quite diverse preileal starch digestibility has been measured even from the same working group ([26Kleffken D. Praeileale Verdauung von Getreidestarke (Gerste / Mais) in Abhängigkeit von Zubereitung, Rauhfutterangebot und Amylasezusatz beim Pferd. Hannover, Germany: Tierärztliche Hochschule 1993.Thesis]: 21.5%, 2 g starch/kg BW [27Heintzsch A, Walther S, Schenkel H, Romanowski K, Zeyner A. Scanning electron microscopic examination of different varieties of oat grains in comparison with the analyzed degree of starch breakdown and glycaemic responses in horses. IJSRSET 1995; 1(2): 110-3.]: 75.3%, 1.3 g starch/kg BW). Despite there was a remarkable difference between the quantity of starch fed per kg of BW in these studies it is rather unlikely that the huge difference in the preileal digestibility of starch can be fully explained by this. It might also be possible that various genotypes of cereal grains characterized by differences in their physico-chemical composition [28Bochnia M, Walther S, Schenkel H, Romanowski K, Zeyner A. Scanning electron microscopic examination of different varieties of oat grains in comparison with the analyzed degree of starch breakdown and glycaemic responses in horses. IJSRSET 2015; 1(2): 110-3.], the ratio of amylose to amylopectin and the content of particular nutrients and anti-nutritional factors [29Rodehutscord M, Rückert C, Maurer HP, et al. Variation in chemical composition and physical characteristics of cereal grains from different genotypes. Arch Anim Nutr 2016; 70(2): 87-107.
[http://dx.doi.org/10.1080/1745039X.2015.1133111] ] are responsible for the availability of starch. To prove this hypothesis it would be necessary to attribute further experimental results in this field not only to the type and amount of cereal grains, but also to its genotype. Furthermore, it would be helpful to characterize the grain in question by more sophisticated methods of feed analysis.
In the recent study, the AUCins also reflects the known superiority of oats compared to maize. Such as for AUCglu, the AUCins was quite similar for oats and barley. For the latter, however, there was a numeric superiority of oats resulting in a quotient of the AUCins:AUCglu being clearly higher with oats (8 µU/ml:mmol/l) than both barley and maize grains (6 µU/ml:mmol/l). The reason for the particular insulin stimulating effect of oats and its particular ingredients is not obvious to the authors’ opinion. The effect might however be at least in part an explanation for the anecdotal, particular encouraging impact of oat grains on the horses’ behavior, because higher levels of plasma insulin stimulate the influx of tryptophan into the brain ([30Daniel PM, Love ER, Moorhouse SR, Pratt OE. The effect of insulin upon the influx of tryptophan into the brain of the rabbit. J Physiol 1981; 312: 551-62.
[http://dx.doi.org/10.1113/jphysiol.1981.sp013643] ]: rabbits) and thus the metabolism of serotonin.
In the recent study, raising quantities of starch did not disproportionately or at least linearly increase the glycaemic and insulinaemic response, respectively. This was irrespective of the starch source. The significantly highest glycaemic reaction and, in terms of numeric differences, insulinaemic response, too, was measured with 1 g starch/kg of BW and meal. In contrast, feeding of a compound feed containing a remarkable proportion of thermal processed starch, which might have been highly available, demonstrated that starch quantities above 0.8 g/kg BW led to disproportionate glycaemic and insulinaemic responses in horses [13Vervuert I, Voigt K, Hollands T, Cuddeford D, Coenen M. Effect of feeding increasing quantities of starch on glycaemic and insulinaemic responses in healthy horses. Vet J 2009; 182(1): 67-72.
[http://dx.doi.org/10.1016/j.tvjl.2008.04.011] ]. Despite it can be assumed that the oat starch in the recent study was also highly available, such a disproportionate effect of higher quantities of starch still staid out. It was instead obvious that the highest concentrations of insulin, but not glucose, were reserved for oat grains. According to [13Vervuert I, Voigt K, Hollands T, Cuddeford D, Coenen M. Effect of feeding increasing quantities of starch on glycaemic and insulinaemic responses in healthy horses. Vet J 2009; 182(1): 67-72.
[http://dx.doi.org/10.1016/j.tvjl.2008.04.011] ], the compound feed contained additional soluble carbohydrates from other sources as cereal grains such as fruits and thermal treated peas with a resulting total sugar content of 68 g/kg DM being somewhat higher than in the recent study (34 – 47 g/kg DM). These alternative sources of highly available carbohydrates and the fact that obviously a remarkable proportion of the ingredients of the compound feed in question underwent thermal processing might be responsible for the cited disproportionate glycaemic and insulinaemic response when more than 0.8 g starch/kg BW and meal were fed [13Vervuert I, Voigt K, Hollands T, Cuddeford D, Coenen M. Effect of feeding increasing quantities of starch on glycaemic and insulinaemic responses in healthy horses. Vet J 2009; 182(1): 67-72.
[http://dx.doi.org/10.1016/j.tvjl.2008.04.011] ]. The not exactly known responsible factors did not act in the described sense under the conditions of the recent study. In the recent study however no biphasic insulin response (first phase: storage-limited model [31Straub SG, Sharp GW. Glucose-stimulated signaling pathways in biphasis insulin secretion. Diabetes Metab Res Rev 2002; 18: 451-63.
[http://dx.doi.org/10.1002/dmrr.329] ]; second phase: signal-limited pool model [32Nunemaker CS, Wasserman DH, McGuiness OP, Sweet IR, Taegues JC, Satin LS. Insulin secretion in the conscious mouse is biphasic and pulsatile. Am JEndocrinol Metab 2006; 290: E523-9.]) occurred because the measuring period was limited to 120 min ppr. only, which was obviously long enough to come near the first peak, but would not have been sufficient to characterize any hours lasting second phase increase. Studies on the ppr. glycaemic and insulinaemic responses covering ppr. periods of 300 min and more did however not indicate the occurrence of a second phase type of insulin increase following ingestion of whole, crushed or ground cereal grains [10Vervuert I, Coenen M, Bothe C. Effects of oat processing on the glycaemic and insulin responses in horses. J Anim Physiol Anim Nutr (Berl) 2003; 87: 96-104.
[http://dx.doi.org/10.1046/j.1439-0396.2003.00420.x] -12Vervuert I, Coenen M, Bothe C. Glycaemic and insulinaemic responses to mechanical or thermal processed barley in horses. J Anim Physiol Anim Nutr (Berl) 2007; 91: 263-8.
[http://dx.doi.org/10.1111/j.1439-0396.2007.00703.x] , 25Zeyner A, Hoffmeister C, Einspanier A, Gottschalk J, Lengwenat O, Illies M. Glycaemic and insulinaemic response of Quarter Horses to concentrates high in fat and low in soluble carbohydrates. Equine Vet J Suppl 2006; 36: 643-7.
[http://dx.doi.org/10.1111/j.2042-3306.2006.tb05619.x] ].
There is though evidence that the glycemic and insulinaemic response of a second starchy meal per day might be lower than the first one in horses, at least following intake of a compound feed [33Karasua GK, Krabbenborg R, Einspanier A, Vervuert I. Insulinaemic and glycaemic responses to a second meal of a fibre- or starch-enriched compound feed in healthy horses. Vet J 2015; 204: 220-2.
[http://dx.doi.org/10.1016/j.tvjl.2015.01.027] ], which in turn may in part be attributed to such long lasting endocrine stimulation. Furthermore, the cereal grain was fed in the recent study 60 min after a hay meal. This was done with respect to maintain gut health [34Zeyner A, Geißler C, Dittrich A. Effects of hay intake and feeding sequence on variables in faeces and faecal water (dry matter, pH value, organic acids, ammonia, buffering capacity) of horses. J Anim Physiol Anim Nutr (Berl) 2004; 88: 7-19.
[http://dx.doi.org/10.1111/j.1439-0396.2004.00447.x] ] and for reasons of standardization and may have altered digesta passage rate and mitigated ppr. responses following the starchy meal.
Particular interesting in the context is the obvious high potential of starch form oat grains to be broken down to glucose and being absorbed in the small intestine despite oat starch granules are probably easy to attack by both microbial and body own enzymes and thus would have disappeared to a large degree already in the stomach of the horse. There is clear evidence that starch and other soluble carbohydrates are to a remarkable degree fermented already in the stomach and the small intestine, respectively [35Coenen M, Mößeler A, Vervuert I. Fermentative gases in breath indicate the Inulin and starch start to be degraded by microbial fermentation in the stomach and small intestine of the horse in contrast to Pectin and Cellulose. J Nutr 2006; 136: 2108S-10S.-39Strauch S, Wichert B, Greef JM, Hillegeist D, Zeyner A, Liesegang A. Evaluation of an in vitro model, simulating equine foregut digestion and the influence of acidity on protein and fructan degradation in the horse´s stomach. J Ani Phys Ani Nut(in print) ]. Whether the starch granules for the moment being protected by the oat grains’ inert spleen, fat or other yet unknown factors needs to be investigated in upcoming studies.
CONCLUSION
Crushed oat and barley grains are ingested faster than cracked maize grains. Crushed oat grains were superior in both the glycaemic and inulinaemic response relative to cracked maize grains in the ppr. raising phase. The absence of any superiority of oat grains in this concern compared to crushed barley grains is unexpected on the contrary. It can be speculated that influences of the genotype of the individual type of cereal grains might have a not yet sufficiently considered impact, which need to be taken into account in further studies. The insulinaemic response to a defined glucose increase seems nevertheless particularly pronounced with oats.
The impact of high insulin levels on the tryptophan influx into the brain, with possible consequences for the serotonin metabolism [40Stracke J, Otten W, Tuchscherer A, Wittham M, et al. Dietary tryptophan supplementation and effective state in pigs. J Vet Beh 2017; 69: 514-23.
[http://dx.doi.org/10.1016/jveb.2017.03.009] -41Stracke J, Otten W, Tuchscherer A, Puppe B, Düpjan S. Serotonin depletion induces pessimistic-like behaviour in a cognitive bias paradigm in pigs. Phy and Beh 2017; 174: 18-26.], might be an explanation for the anecdotal encouraging effect of oat grains on the horses’ behavior, at least in part. From 0.8 to 2 g/kg body weight increasing quantities of starch from crushed oats and barley as well as cracked maize grains fed as the only starch source seem not to induce a disproportionate or at least linear increase of plasma glucose and insulin at least in a rising phase until 120 min ppr., respectively.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors, A. Zeyner, K. Romanowski, A. Orgis, A. Vernunft, J. Gottschalk, A. Einspanier, and G. Koeller, declare they have no financial and non-financial conflict of interest. The authors’ freedom to design, conduct, interpret and publish research is not compromised by any controlling sponsor as a condition of review or publication.
ACKNOWLEDGEMENTS
The project was supported by funds of the Federal Ministry of Food, Agriculture and Consumer Protection (BMELV) based on a decision of the Parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food (BLE) under the innovation support program (Project GrainUp, grant number 2813801510).
The study was performed in accordance with the Federation of Animal Science Societies’ Animal Care Guidelines [42Stracke J, Otten W, Tuchscherer A, Wittham M, et al. Federation of Animal Science (transferred to American Dairy Science Organisation, American Society of Animal Science and Poultry Association, 2016), Eds Guide for the care and use of agricultural animals in research and teaching (FASS 2010 Ag Guide) 3rd ed. 2010.
[PMID: 978-1-884706-11-0] ]. The study was approved by the animal welfare committee of Mecklenburg-Western Pomerania (LALLFM-V/TSD/7221.3.1.1-036/11).
The authors are grateful to Lena Pape and Heino Marx for animal care and technical assistance during the experiment as well as Diana Schmiedel and Angela Schmidt for performing feed analysis.
REFERENCES
| [1] | Zeyner A. Energy providing nutrient sources. In: Saastamoinen MT, Martin-Rosset W, Eds. Nutrition of the exercising horse EAAP publication no 125. Wageningen, The Netherlands: Wageningen Academic Publishers 2008; pp. 277-94. |
| [2] | Radicke S, Landes E, Kienzle E, Meyer H. Aktivität der Amylase im Darmkanal des Pferdes in Abhängigkeit von der Futterart Pferdeheilkunde. Sonderausgabe 1992; pp. 99-102. |
| [3] | Garner HE, Coffman JR, Hahn AW, Hutcheson DP, Tumbleson ME. Equine laminitis of alimentary origin: an experimental model. Am J Vet Sci 1975; 30: 441-4. |
| [4] | Meyer H, Radicke S, Kienzle E, Wilke S, Kleffken D, Illenseer M. Investigations on preileal digestion of starch from grain, potato and manioc in horses. J Vet Med A 1995; 42: 371-81. [http://dx.doi.org/10.1111/j.1439-0442.1995.tb00389.x] |
| [5] | Julliand V, De Frombelle A, Varloud M. Starch digestion in horses: The impact of feed processing. Livest Sci 2006; 100: 44-52. [http://dx.doi.org/10.1016/j.livprodsci.2005.11.001] |
| [6] | Kienzle E, Pohlenz J, Radicke S. Morphology of starch digestion in the horse. J Vet Med A 1997; 44: 207-21. [http://dx.doi.org/10.1111/j.1439-0442.1997.tb01103.x] |
| [7] | Bochnia M, Walther S, Schenkel H, Romanowski K, Zeyner A. Comparison of scanning electron microscopic examination of oats, barley and maize grains with the analyzed degree of starch breakdown and glycaemic responses in horses. IJSRSET 2015; 1(2): 81-4. |
| [8] | Luthersson N, Hou Nielsen K, Harris P, Parkin TD. Risk factors associated with equine gastric ulceration syndrome (EGUS) in 201 horses in Denmark. Equine Vet J 2009; 41: 625-30. [http://dx.doi.org/10.2746/042516409X441929] |
| [9] | Treiber KH, Boston RC, Kronfeld DS, Staniar WB, Harris PA. Insulin resistance and compensation in Thoroughbred weanlings adapted to high glycemic meals. J Anim Sci 2005; 83: 2357-64. [http://dx.doi.org/10.2527/2005.83102357x] |
| [10] | Vervuert I, Coenen M, Bothe C. Effects of oat processing on the glycaemic and insulin responses in horses. J Anim Physiol Anim Nutr (Berl) 2003; 87: 96-104. [http://dx.doi.org/10.1046/j.1439-0396.2003.00420.x] |
| [11] | Vervuert I, Coenen M, Bothe C. Effects of corn processing on the glycaemic and insulinaemic responses in horses. J Anim Physiol Anim Nutr (Berl) 2004; 88: 348-55. [http://dx.doi.org/10.1111/j.1439-0396.2004.00491.x] |
| [12] | Vervuert I, Coenen M, Bothe C. Glycaemic and insulinaemic responses to mechanical or thermal processed barley in horses. J Anim Physiol Anim Nutr (Berl) 2007; 91: 263-8. [http://dx.doi.org/10.1111/j.1439-0396.2007.00703.x] |
| [13] | Vervuert I, Voigt K, Hollands T, Cuddeford D, Coenen M. Effect of feeding increasing quantities of starch on glycaemic and insulinaemic responses in healthy horses. Vet J 2009; 182(1): 67-72. [http://dx.doi.org/10.1016/j.tvjl.2008.04.011] |
| [14] | Gesellschaft für Ernährungsphysiologie, Ed Energie- und Nährstoffbedarf landwirtschaftlicher Nutztiere 11, Empfehlungen zur Energie- und Nährstoffversorgung von Pferden. Frankfurt am Main: DLG-Verlag 2014. |
| [15] | Kienzle E, Schramme S. Beurteilung des Ernährungszustandes mittels Body Condition Scores und Gewichtsschätzung beim adulten Warmblutpferd. Pferdeheilkunde 2004; 20(6): 517-24. [http://dx.doi.org/10.21836/PEM20040604] |
| [16] | Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten, Ed Die chemische Untersuchung von Futtermitteln Loseblattsammlung mit Ergänzungslieferungen. Darmstadt: VDLUFA-Verlag 1976; III.Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch) |
| [17] | Zeyner A, Quellmalz S, Dill J, Lengwenat O. Reproducibility of data on the chewing activity of horses measured by CARA. Proceedings of the 13th Congress of the European Society of Veterinary and Comparative Nutrition (ESVCN). 2009 Oct 15-17; 116. |
| [18] | Zeyner A, Gefrom A, Hillegeist D, Sommer M, Greef JM. Contribution to the method of sugar analysis in legume grains for ensiling – a pilot study. IJSRSET 2015; 1(2): 74-80. |
| [19] | Köller G, Gieseler T, Schusser GF. Hämatologische und blutchemische Referenzbereiche bei Pferden unterschiedlicher Rasse und Altersgruppen basierend auf neuesten labordiagnostischen Methoden. Pferdeheilkunde 2014; 30(4): 381-93. |
| [20] | Gottschalk J, Einspanier A, Ungemach FR, Abraham G. Influence of topical dexamethasone applications on insulin, glucose, thyroid hormone and cortisol levels in dogs. Res Vet Sci 2011; 90: 491-7. [http://dx.doi.org/10.1016/j.rvsc.2010.06.026] |
| [21] | McLean BM, Hyslopp JJ, Longland AC, Cuddeford D, Hollands T. Physical processing of barley and its effects on intra-caecal fermentation patterns in ponies. Anim Feed Sci Technol 2000; 85: 79-87. [http://dx.doi.org/10.1016/S0377-8401(00)00132-2] |
| [22] | van Weyenberg S, Buyse J, Janssens GP. Digestibility of a complete ration in horses fed once ore three times a day and correlation with key blood parameters. Vet J 2007; 173: 311-6. [http://dx.doi.org/10.1016/j.tvjl.2005.08.011] |
| [23] | Kronfeld DS, Treiber KH, Hess TM, Boston RC. Insulin resistance in the horse: definition, detection, and dietetics. J Anim Sci 2005; 83: 1-10. |
| [24] | Dunbar LK, Mielnicki KA, Dembek KA, Toribio RE, Burns TA. Evaluation of four diagnostic tests for insulin dysregulation in adult light-breed horses. J Vet Intern Med 2016; 30: 885-91. [http://dx.doi.org/10.1111/jvim.13934] |
| [25] | Zeyner A, Hoffmeister C, Einspanier A, Gottschalk J, Lengwenat O, Illies M. Glycaemic and insulinaemic response of Quarter Horses to concentrates high in fat and low in soluble carbohydrates. Equine Vet J Suppl 2006; 36: 643-7. [http://dx.doi.org/10.1111/j.2042-3306.2006.tb05619.x] |
| [26] | Kleffken D. Praeileale Verdauung von Getreidestarke (Gerste / Mais) in Abhängigkeit von Zubereitung, Rauhfutterangebot und Amylasezusatz beim Pferd. Hannover, Germany: Tierärztliche Hochschule 1993.Thesis |
| [27] | Heintzsch A, Walther S, Schenkel H, Romanowski K, Zeyner A. Scanning electron microscopic examination of different varieties of oat grains in comparison with the analyzed degree of starch breakdown and glycaemic responses in horses. IJSRSET 1995; 1(2): 110-3. |
| [28] | Bochnia M, Walther S, Schenkel H, Romanowski K, Zeyner A. Scanning electron microscopic examination of different varieties of oat grains in comparison with the analyzed degree of starch breakdown and glycaemic responses in horses. IJSRSET 2015; 1(2): 110-3. |
| [29] | Rodehutscord M, Rückert C, Maurer HP, et al. Variation in chemical composition and physical characteristics of cereal grains from different genotypes. Arch Anim Nutr 2016; 70(2): 87-107. [http://dx.doi.org/10.1080/1745039X.2015.1133111] |
| [30] | Daniel PM, Love ER, Moorhouse SR, Pratt OE. The effect of insulin upon the influx of tryptophan into the brain of the rabbit. J Physiol 1981; 312: 551-62. [http://dx.doi.org/10.1113/jphysiol.1981.sp013643] |
| [31] | Straub SG, Sharp GW. Glucose-stimulated signaling pathways in biphasis insulin secretion. Diabetes Metab Res Rev 2002; 18: 451-63. [http://dx.doi.org/10.1002/dmrr.329] |
| [32] | Nunemaker CS, Wasserman DH, McGuiness OP, Sweet IR, Taegues JC, Satin LS. Insulin secretion in the conscious mouse is biphasic and pulsatile. Am JEndocrinol Metab 2006; 290: E523-9. |
| [33] | Karasua GK, Krabbenborg R, Einspanier A, Vervuert I. Insulinaemic and glycaemic responses to a second meal of a fibre- or starch-enriched compound feed in healthy horses. Vet J 2015; 204: 220-2. [http://dx.doi.org/10.1016/j.tvjl.2015.01.027] |
| [34] | Zeyner A, Geißler C, Dittrich A. Effects of hay intake and feeding sequence on variables in faeces and faecal water (dry matter, pH value, organic acids, ammonia, buffering capacity) of horses. J Anim Physiol Anim Nutr (Berl) 2004; 88: 7-19. [http://dx.doi.org/10.1111/j.1439-0396.2004.00447.x] |
| [35] | Coenen M, Mößeler A, Vervuert I. Fermentative gases in breath indicate the Inulin and starch start to be degraded by microbial fermentation in the stomach and small intestine of the horse in contrast to Pectin and Cellulose. J Nutr 2006; 136: 2108S-10S. |
| [36] | Ince JC, Longland AC, Moore-Colyer MJ, Harris PA. In vitro degradation of grass fructan by equid gastrointestinal digesta. Grass Forage Sci 2013; 69: 514-23. [http://dx.doi.org/10.1111/gfs.12061] |
| [37] | Glatter M, Hillegeist D, Bochnia M, et al. Variation of carbohydrate content and degree of polymerization along the equine gastrointestinal tract. Proceedings of the 70th Conference of the Society of Nutrition Physiology. 2016 March 8-10; Hannover, Germany. 2016. |
| [38] | Glatter M, Wiedner K, Hirche F, et al. Fermentation characteristics along the gastrointestinal tract after feeding of Jerusalem artichoke meal to adult healthy warmblood horses. J Ani Nut 2016; 1(3:16): 1-11. Available from: http://animalnutrition.imedpub.com [http://dx.doi.org/10.21767/2572-5459.100016] |
| [39] | Strauch S, Wichert B, Greef JM, Hillegeist D, Zeyner A, Liesegang A. Evaluation of an in vitro model, simulating equine foregut digestion and the influence of acidity on protein and fructan degradation in the horse´s stomach. J Ani Phys Ani Nut(in print) |
| [40] | Stracke J, Otten W, Tuchscherer A, Wittham M, et al. Dietary tryptophan supplementation and effective state in pigs. J Vet Beh 2017; 69: 514-23. [http://dx.doi.org/10.1016/jveb.2017.03.009] |
| [41] | Stracke J, Otten W, Tuchscherer A, Puppe B, Düpjan S. Serotonin depletion induces pessimistic-like behaviour in a cognitive bias paradigm in pigs. Phy and Beh 2017; 174: 18-26. |
| [42] | Stracke J, Otten W, Tuchscherer A, Wittham M, et al. Federation of Animal Science (transferred to American Dairy Science Organisation, American Society of Animal Science and Poultry Association, 2016), Eds Guide for the care and use of agricultural animals in research and teaching (FASS 2010 Ag Guide) 3rd ed. 2010. [PMID: 978-1-884706-11-0] |