- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Ornithology Journal
(Discontinued)
ISSN: 1874-4532 ― Volume 13, 2020
Different Messages are Transmitted by Individual Duet Contributions and Complete Duets in a Species with Highly Overlapped Duets
Luis Sandoval1, 2, *, Roselvy Juárez1, Mauricio Villarreal1, 2
Abstract
Background:
Duet function hypotheses have been mostly studied in bird species that produce duets with male and female solo songs. However, in order to understand if patterns of duet function are similar across all duetting species, it is highly necessary to test the duet function hypotheses in species that produce duets with vocalizations other than solo songs.
Objective:
We studied the responses of territorial pairs to each sex’s individual duet contribution and complete duets in a species that produces duets with a vocalization other than male and female solo songs.
Methods:
We conducted a playback experiment where we presented duet contributions of each sex to three populations of White-eared Ground-sparrows (Melozone leucotis) in Costa Rica, during this species’ breeding season in 2016.
Results:
The responses to complete duets were stronger than those to each sex’s duet contribution, suggesting that complete duets and each sex’s duet contribution have different functions. Complete duets are used to protect resources from intruders (supporting the resource defense hypothesis), and to prevent the partner from being usurped by intruders (supporting the mate-guarding hypothesis). Males used solo songs in response to female duet contributions, and this may work to attract intruder females (increasing the probability of extra-pair copulation). Males also use solo songs in response to male duet contributions, which may work as a signal to repel intruder males and guard their female. In this case, where mate attraction occurs with a completely different type of vocalization than used for duetting, we found a clear pattern of a double agenda for males when a territorial intrusion occurs.
Conclusions:
This study provides strong support for the dual function hypothesis in duets and reveals conflicting selective pressures between pair members relative to each hypothesis.
Article Information
Identifiers and Pagination:
Year: 2018Volume: 11
First Page: 56
Last Page: 67
Publisher Id: TOOENIJ-11-56
DOI: 10.2174/1874453201811010056
Article History:
Received Date: 22/6/2018Revision Received Date: 1/10/2018
Acceptance Date: 21/10/2018
Electronic publication date: 22/11/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Escuela de Biología, Universidad de Costa Rica, Montes de Oca, San José, CP-11501-2060, Costa Rica; Tel: (506)25118681; E-mail: biosandoval@hotmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 22-6-2018 |
Original Manuscript | Different Messages are Transmitted by Individual Duet Contributions and Complete Duets in a Species with Highly Overlapped Duets | |
1. INTRODUCTION
Vocal displays coordinated in time and/or frequency between two individuals of the same or opposite sex are called duets and occur in diverse taxa [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] ]. Among all taxonomical groups that produce duets (e.g., insects, anurans,birds, and mammals) [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] -3Burton JA, Nietsch A. Geographic variation in duet songs of Sulawesi tarsiers: Evidence for new cryptic species in south and southeast Sulawesi. Int J Primatol 2010; 31: 1123-46.
[http://dx.doi.org/10.1007/s10764-010-9449-8] ], birds are the group where this vocal behavior is most commonly reported and studied [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] , 4Hall ML In Naguib M, Janik VM. Ed Advances in the Study of Behavior, 2009; 40: 67-121.]. This is because duets occur in several different related and non-related bird species in different geographical regions. Most duetting bird species are found in tropical regions rather than temperate zones because in tropical regions, female song occurs most commonly [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] , 5Benedict L. Occurrence and life history correlates of vocal duetting in North American passerines. J Avian Biol 2008; 39: 57-65. a
[http://dx.doi.org/10.1111/j.0908-8857.2008.04103.x] -7Odom KJ, Hall ML, Riebel K, Omland KE, Langmore NE. Female song is widespread and ancestral in songbirds. Nat Commun 2014; 5: 3379.
[http://dx.doi.org/10.1038/ncomms4379] [PMID: 24594930] ], and this is a requirement for vocal duets in the majority of species [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] , 4Hall ML In Naguib M, Janik VM. Ed Advances in the Study of Behavior, 2009; 40: 67-121.]; with the exception of Chiroxiphia manakins, another group of tropical species, duets are produced by two males [8McDonald DB, Potts WK. Cooperative display and relatedness among males in a lek-mating bird. Science 1994; 266(5187): 1030-2.
[http://dx.doi.org/10.1126/science.7973654] [PMID: 7973654] , 9Maynard DF, Ward KA, Doucet SM, Mennill DJ. Calling in an acoustically competitive environment: Duetting male long-tailed manakins avoid overlapping neighbours but not playback-simulated rivals. Anim Behav 2012; 84: 563-73.
[http://dx.doi.org/10.1016/j.anbehav.2012.06.008] ].
In birds, there are 12 proposed hypotheses for duet function, which can be split into two main groups [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] , 4Hall ML In Naguib M, Janik VM. Ed Advances in the Study of Behavior, 2009; 40: 67-121.]. The first group of hypotheses, where pair members compete for their own benefit (intrapair conflicts), include mate guarding, paternity guarding, extra-pair mating, and change to partners [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] , 10Dahlin CR, Benedict L. Angry birds need not apply: A perspective on the flexible form and multifunctionality of avian vocal duets. Ethology 2014; 120: 1-10.
[http://dx.doi.org/10.1111/eth.12182] ]. The second group of hypotheses, where pair members cooperate for mutual benefits [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] , 4Hall ML In Naguib M, Janik VM. Ed Advances in the Study of Behavior, 2009; 40: 67-121.], include joint resource defense, maintaining the pair bond, protection from predators, and ensuring reproductive synchrony [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] ]. Although the evidence from experimental studies has supported both groups of functions and all hypotheses proposed, it has also shown that duets are sometimes multifunctional within species [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] , 4Hall ML In Naguib M, Janik VM. Ed Advances in the Study of Behavior, 2009; 40: 67-121.]. For example, duet studies of Rufous-and-white Wren (Thryophilus rufalbus) [11Mennill DJ, Vehrencamp SL. Context-dependent functions of avian duets revealed by microphone-array recordings and multispeaker playback. Curr Biol 2008; 18(17): 1314-9.
[http://dx.doi.org/10.1016/j.cub.2008.07.073] [PMID: 18771924] , 12Kahn ZA, Moser-Purdy CE, Mennill DJ. Sing and do not stray: Male Rufous-and-white Wrens use duets and physical behaviours to guard their mates. Anim Behav in press
[http://dx.doi.org/10.1016/j.anbehav.2018.07.005] ] support the resource defense, mate guarding, and recognition and contact hypotheses. In Magpie-larks (Grallina cyanoleuca) and California Towhees (Melozone crissalis), duets are used in resource defense and mate guarding [13Hall ML. The function of duetting in magpie-larks: conflict, cooperation, or commitment? Anim Behav 2000; 60(5): 667-77.
[http://dx.doi.org/10.1006/anbe.2000.1517] [PMID: 11082237] -16].
However, the majority of studies on duet function have been conducted in bird species that produce duets using male and female solo songs (e.g., antbirds, wrens, fairy-wrens, boubous) [4Hall ML In Naguib M, Janik VM. Ed Advances in the Study of Behavior, 2009; 40: 67-121., 5Benedict L. Occurrence and life history correlates of vocal duetting in North American passerines. J Avian Biol 2008; 39: 57-65. a
[http://dx.doi.org/10.1111/j.0908-8857.2008.04103.x] , 10Dahlin CR, Benedict L. Angry birds need not apply: A perspective on the flexible form and multifunctionality of avian vocal duets. Ethology 2014; 120: 1-10.
[http://dx.doi.org/10.1111/eth.12182] ]. However, in order to understand if patterns of duet function are similar across all duetting species, it is highly necessary to test the duet function hypotheses in species that produce duets with vocalizations other than solo songs (e.g., towhees, ground-sparrows, or finches) [17 - 19]. If duets show similar functions in species that produce duets using solo songs, as well as those that duet with other types of vocalizations, this would provide direct evidence of consistency in duet function across bird species. In those species that do not use solo songs to create duets, females apparently lack the capacity to produce male solo songs [18, 20]. Therefore, another type of vocalization, differing structurally from the solo song, is produced by both sexes to create duets [5Benedict L. Occurrence and life history correlates of vocal duetting in North American passerines. J Avian Biol 2008; 39: 57-65. a
[http://dx.doi.org/10.1111/j.0908-8857.2008.04103.x] , 19, 20]. Additionally, vocalizations used to produce duets are rarely produced alone in those species and a single individual contribution to the duets produces an incomplete duet [18, 20], confirming that the main purpose of these vocalizations is to produce duets.
The main objective of this study is to test if territorial pairs respond differently to each sex’s duet contributions and complete duets in a bird species that produces duets with a vocalization other than solo songs. According to hypotheses of duet function [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] ], we predict that (1) if duets function in joint resource defense, duet stimuli would evoke a more intense response from territorial pairs than each sex’s contribution separately, because paired intruders represent a greater threat to resources than a solitary intruder [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] , 4Hall ML In Naguib M, Janik VM. Ed Advances in the Study of Behavior, 2009; 40: 67-121.]. (2) If duets function in mate guarding (intrasexual conflict), each sex’s contribution would evoke a more intense response than a complete duet, because a solitary intruder represents more threat to a same-sex individual than paired intruders, given the increased probability that same-sex rivals may pair or copulate with their partner [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] , 4Hall ML In Naguib M, Janik VM. Ed Advances in the Study of Behavior, 2009; 40: 67-121.]. In this case, we would expect more intense responses towards same-sex duet contributions than towards opposite-sex contributions, because duet contributions are used for mate guarding and preventing extra-pair mating.
2. METHODS
We conducted this study in three populations of White-eared Ground-sparrows Melozone leucotis in the Central Valley of Costa Rica: (1) Getsemani, Heredia province (10°01’N, 84°05’W, 1350 m); (2) Universidad de Costa Rica Campus, San Jose province (09°56’N, 84°05’W, 1200 m); and (3) Jardín Botánico Lankester, Cartago province (9°50’N, 83°53’W, 1370 m). The study was conducted from 2 to 6 May 2016 during this species’ breeding season, and from 0600 to 0900 hrs when this species is most vocally active [20]. The number of pairs studied varied between populations: 13 at Universidad de Costa Rica campus, 10 at Getsemaní, and 9 at Jardín Botánico Lankester. In all 32 pairs, one or both individuals were color-banded, allowing individual and sex recognition. White-eared Ground-sparrows produce duets using vocalizations other than male solo songs [20], contrary to the majority of studied species that produce duets [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] , 4Hall ML In Naguib M, Janik VM. Ed Advances in the Study of Behavior, 2009; 40: 67-121.].
2.1. Playback Stimuli
We create a single-channel stimulus using recordings obtained from previous years in the three populations. Recordings were obtained using a solid-state recorder (Marantz PMD661; sampling rate: 44.1 kHz; accuracy: 16-bit; file format: WAVE) with a Sennheiser K6/ME66 shotgun microphone. Stimuli were created by isolating vocalizations (duets, male contributions, and female contributions, (Fig. 1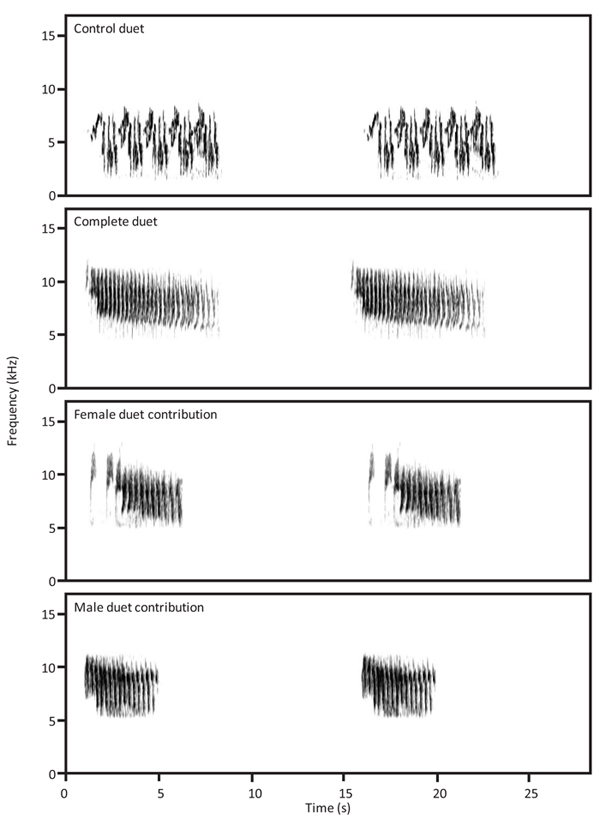 )) with a high signal-to-noise ratio which was not overlapped with other sounds. Selected male and female contributions and duets were filtered to delete background noise outside of the target vocalization’s frequency range using the FFT filter in Adobe Audition 1.0 (Adobe System Incorporated); using the same software, we normalized the energy in all stimuli to -1 dB.
)) with a high signal-to-noise ratio which was not overlapped with other sounds. Selected male and female contributions and duets were filtered to delete background noise outside of the target vocalization’s frequency range using the FFT filter in Adobe Audition 1.0 (Adobe System Incorporated); using the same software, we normalized the energy in all stimuli to -1 dB.
Male and female contributions to duets were obtained when individuals of each sex sang their contributions and the other sex did not respond to create the duet (< 29% of occasions when pair members produced duet vocalizations) [20]. We only used male and female contributions to duets when we could visually confirm the identity of the singing individual because both sexes can produce both contributions and regularly sing from hidden perches [20]; therefore, identification based only on duet vocalizations is not possible. We obtained two male and two female duet contributions from each population, for a total of six per sex. This low sample size is due to the low occurrence of lone duet contributions and the challenges related to confirming the sex of the individual that produced them [20]. Therefore, each pair received playback of a duet contribution from another population to minimize the effects of familiarity with individuals. For complete duets, we used six different duets (two per population) to match our sample size of duet contributions and avoid differences in responses caused by using more different types of duet stimuli than individual duet contributions. As explained above, each pair received playback of a complete duet from another population. Complete duets of White-eared Ground-sparrows show low pair distinctiveness, as shown in a previous study which analyzed 582 duets [20]; therefore, we are confident that the small set of stimuli of complete duets we used are representative of this species’ duet variation. Additionally, we used six duets from Cabanis's Wren (Cantorchilus modestus) as a control, because it is a species that shares habitat and territories with White-eared Ground-sparrows but is not a competitor due to differences in diet and breeding requirements.
We broadcasted complete duets, each sex’s individual contributions, and control duets at a rate of four vocalizations per minute inside White-eared Ground-sparrow territories at 5-10 m from one territory edge. Each playback trial involved 2 minutes of vocalizations followed by 5 minutes of silence. Ground-sparrows' response behavior was observed during the 2 minutes of playback plus the first 3 minutes of the silent period. We used the last 2 minutes of the silent period to allow the focal pair to resume their pre-playback activities (e.g., leaving the area around the speaker, foraging, or moving inside the territory). The four stimuli were presented in quick succession during a single day; an approach used in other playback experiments with territorial birds [21-24]. Stimulus order was selected following a balanced design, where each stimulus occurs in each possible position and in the same proportion (i.e., duets occurred in the first position in 7 pairs, in the second position in 8 pairs, in the third position in 7 pairs, and in the fourth position in 10 pairs).
We used an Anchor Audio Minivox loudspeaker mounted at a height of 1.25 m attached to an iPod Nano portable audio player to conduct the playbacks. The observer that conducted the playback was located at 8 m from the loudspeaker observing and recording the birds’ response behaviors. Playbacks were conducted at a constant volume of 80 dB SPL, measured at 1 m from the speaker using a Sper Scientific digital sound level meter (model NIB -850014, using fast response and A-weighting). This amplitude is similar to the amplitude of duets heard in the field at the same distance and is the same amplitude used in another playback experiment with this species [24, 25].
2.2. Response Measures
We measured five behavioral responses during playback experiments: (1) the latency of the first vocalization (in seconds); if the pair did not vocalize, we assigned a value of 301 s; (2) the latency of approach for the first individual of the pair within 3 m from the loudspeaker (in seconds); if no individual approached, we assigned a value of 301 s; (3) total time inside the 3 m radius from the loudspeaker (in seconds); if no individual was within the 3 m radius, we assigned a value of 0 s; (4) total number of vocalizations; and (5) number of individuals of the pair that approached within 3 m from the loudspeaker (0 - 2). We recorded all vocalizations produced in response to the playback during the first 5 minutes of the experiment using a solid-state recorder (Marantz PMD661; sampling rate: 44.1 kHz; accuracy: 16-bit; file format: WAVE) with a Sennheiser K6/ME66 shotgun microphone. For each recorded vocalization, we measured (1) the minimum frequency (in kHz), (2) the maximum frequency (in kHz), (3) the frequency of maximum amplitude (in kHz), (4) the duration (in seconds), and (5) the entropy (in µ), which is the distribution of energy in the sound (low values mean an equal distribution of energy in the sound time and frequency, whereas high values mean an unequal distribution of energy in the sound time and frequency). These measurements were obtained using a combination of the spectrogram (to identify the vocalization), the power spectrum (to measure frequency limits), and the wave spectrum (to measure time limits), in the sound analysis software Raven 1.4 (Cornell Lab of Ornithology, Ithaca, NY, USA). The software configuration followed these values: temporal resolution of 5.8 ms and a Hann window with 256 kHz sampling, and a frequency resolution of 188 Hz with 50% overlap.
2.3. Statistical Analysis
We conducted a Principal Component Analysis (PCA) to reduce our five behavioral responses into two composite responses (principal components with an eigenvalue greater than 1.0) that describe the response behavior of our territorial White-eared Ground-sparrow pairs. The first component explained 51.54% of the original variance of five response variables, and showed a strong relationship with rapid approach to 3 m from the loudspeaker (r = -0.91), more time inside 3 m radius from the loudspeaker (r = 0.86), and more individuals approaching (r = 0.85); but a weak relationship with the time of the first vocalization (r = -0.15) and number of vocalizations (r = 0.06). We called this first principal component “approach response”, where pairs with higher values approached faster, spent more time close to the stimulus, and more individuals approached. The second component explained 22.29% of the original variance of five response variables, and showed a strong relationship with rapid vocalizations (r = -0.77) and number of vocalizations (r = 0.80); but a weak relationship with approach to 3 m from the loudspeaker (r = -0.13), time inside 3 m radius from the loudspeaker (r = 0.06), and the number of individuals approaching (r = 0.17). We called this second principal component “vocal response”, where pairs with higher values vocalized faster and produced more vocalizations. Then, we conducted two linear mixed-effects models to test how the approach and vocal responses varied according to the stimulus used (four levels: complete duet, male or female duet contribution separately, and control). We included pair and playback identity as random factors in our linear mixed-effect models.
We also conducted PCA to reduce the five acoustical measurements of each type of vocalization (i.e., calls, songs, and duets) produced as responses to the playback during the first 5 minutes of the experiment into two composite responses (principal components with an eigenvalue greater than 1.0). These components described the structure of the vocalizations of White-eared Ground-sparrow territorial pairs. Each type of vocalization was analyzed separately, because they are structurally different [20, 24]. The first component for chip calls explained 49.81% of the original variance of the five response variables, and showed a strong relationship with frequency of maximum amplitude (r = 0.91), minimum (r = -0.96), and maximum frequency (r = 0.86); but a weak relationship with duration (r = -0.07) and energy entropy (r = -0.08). The second component explained 25.41% of the original variance of five response variables, and showed a strong relationship with duration (r = 0.80) and energy entropy (r = -0.77); but a weak relationship with frequency of maximum amplitude (r = 0.11), minimum (r = 0.06), and maximum frequency (r = -0.16).
The first component for tseet calls explained 42.20% of the original variance of the five response variables, and showed a strong relationship with duration (r = 0.71), maximum frequency (r = 0.83), and energy entropy (r = 0.92); but a weak relationship with frequency of maximum amplitude (r = 0.05) and minimum frequency (r = -0.10). The second component explained 34.33% of the original variance of five response variables, and showed a strong relationship with frequency of maximum amplitude (r = 0.84) and minimum frequency (r = 0.83); but a weak relationship with duration (r = -0.11), maximum frequency (r = 0.43), and energy entropy (r = -0.13).
The first component for solo songs explained 36.92% of the original variance of the five response variables, and showed a strong relationship with duration (r = 0.86), frequency of maximum amplitude (r = 0.64), and maximum frequency (r = 0.73); but a weak relationship with energy entropy (r = 0.12) and minimum frequency (r = 0.26). The second component explained 28.82% of the original variance of five response variables, and showed a strong relationship with energy entropy (r = -0.83) and minimum frequency (r = 0.84); but a weak relationship with duration (r = 0.15), frequency of maximum amplitude (r = 0.18), and maximum frequency (r = -0.27).
The first component for duets explained 30.04% of the original variance of the five response variables, and showed a strong relationship with maximum frequency (r = 0.82) and energy entropy (r = -0.70); but a weak relationship with duration (r = 0.43), frequency of maximum amplitude (r = 0.32) and minimum frequency (r = -0.04). The second component explained 25.65% of the original variance of five response variables, and showed a strong relationship with duration (r = -0.68), frequency of maximum amplitude (r = 0.67) and minimum frequency (r = 0.60); but a weak relationship with maximum frequency (r = 0.15), and energy entropy (r = 0.14). Then, we conducted two linear mixed-effects models to test whether PC1 and PC2 varied according to the stimulus used (four levels: complete duet, male or female duet contribution separately, and control). We included pair and playback identity as random factors in our linear mixed-effects models.
We used a chi-square goodness-of-fit test to compare if the sex of individuals that approached within 3 m radius from the speaker (i.e., male, female, or both) varied according to stimulus type. We used another chi-square goodness-of-fit test to compare if the type of vocalization (i.e., calls, songs, and duets) produced in response to the stimuli varied according to stimulus type. All tests were two-tailed, and values are reported as means ± SE. All statistical analyses were conducted in JMP (version 10.0; SAS Institute, Cary, NC, U.S.A.).
3. RESULTS
We found that territorial pairs of White-eared Ground-sparrows responded strongly to playbacks of complete duets as well as to playbacks of each sex’s duet contribution, approaching and producing vocalizations as responses. The approach response summarized by the first principal component was similar between complete duets and each sex’s duet contribution, and higher towards all conspecific stimuli than to control duets (F3,6 = 6.39, P = 0.03) (Fig. 2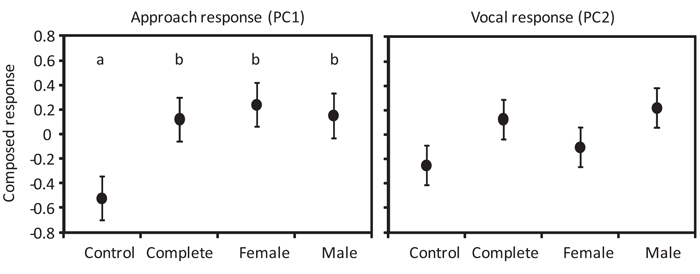 ). The vocal response (PC2) was similar between all playback stimuli (F3,7 = 2.69, P = 0.13) (Fig. 2
). The vocal response (PC2) was similar between all playback stimuli (F3,7 = 2.69, P = 0.13) (Fig. 2 ). Chip calls produced in response to complete duets and male duet contributions had a higher frequency of maximum amplitude and maximum frequency, but lower minimum frequency than those produced in response to control duets and female duet contributions (PC1: F3,6 = 4.31, P < 0.001) (Fig. 3
). Chip calls produced in response to complete duets and male duet contributions had a higher frequency of maximum amplitude and maximum frequency, but lower minimum frequency than those produced in response to control duets and female duet contributions (PC1: F3,6 = 4.31, P < 0.001) (Fig. 3 ). Duration and energy entropy were similar between all stimuli (PC2: F3,7 = 0.58, P=0.65) (Fig. 3
). Duration and energy entropy were similar between all stimuli (PC2: F3,7 = 0.58, P=0.65) (Fig. 3 ). Tseet calls produced in response to female duet contributions had longer duration, and higher maximum frequency and energy entropy than those produced in response to complete duets (PC1: F3,31 = 10.03, P < 0.001) (Fig. 3
). Tseet calls produced in response to female duet contributions had longer duration, and higher maximum frequency and energy entropy than those produced in response to complete duets (PC1: F3,31 = 10.03, P < 0.001) (Fig. 3 ); however, frequency of maximum amplitude and minimum frequency were similar in response to all stimuli (PC2: F3,31 = 1.09, P = 0.59) (Fig. 3
); however, frequency of maximum amplitude and minimum frequency were similar in response to all stimuli (PC2: F3,31 = 1.09, P = 0.59) (Fig. 3 ). Solo songs produced in response to all stimuli showed no differences in duration, frequency of maximum amplitude, and maximum frequency (PC1: F3,6 = 0.12, P = 0.94) (Fig. 3
). Solo songs produced in response to all stimuli showed no differences in duration, frequency of maximum amplitude, and maximum frequency (PC1: F3,6 = 0.12, P = 0.94) (Fig. 3 ); however, solo songs produced in response to female duet contributions had lower minimum frequency and energy entropy compared to those produced in response to control duets (PC2: F3,7 = 85.94, P < 0.001) (Fig. 3
); however, solo songs produced in response to female duet contributions had lower minimum frequency and energy entropy compared to those produced in response to control duets (PC2: F3,7 = 85.94, P < 0.001) (Fig. 3 ). Duets produced in response to all stimuli showed no differences in maximum frequency and energy entropy (PC1: F3,8 = 0.90, P = 0. 84) (Fig. 3
). Duets produced in response to all stimuli showed no differences in maximum frequency and energy entropy (PC1: F3,8 = 0.90, P = 0. 84) (Fig. 3 ), or duration, frequency of maximum amplitude, and minimum frequency (PC2: F3,8 = 0.35, P = 0.81) (Fig. 3
), or duration, frequency of maximum amplitude, and minimum frequency (PC2: F3,8 = 0.35, P = 0.81) (Fig. 3 ).
).
We found differences in the sex of the individuals that approached within a 3 m radius from the speaker according to the playback stimulus used (Χ2 = 15.92, df = 6, P = 0.01). When we played back complete duets, both individuals of the pair were more likely to approach the stimulus than each sex alone (Fig. 4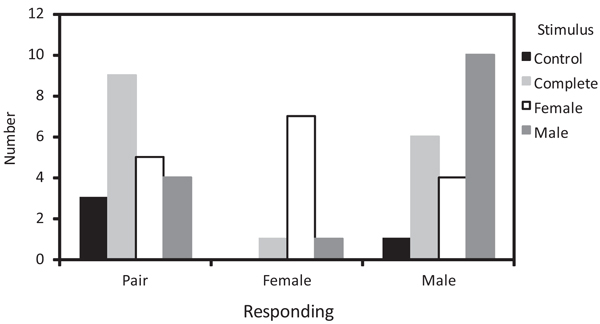 ). When the stimulus was from a female duet contribution, the female of the pair was most likely to approach the stimulus (Fig. 4
). When the stimulus was from a female duet contribution, the female of the pair was most likely to approach the stimulus (Fig. 4 ). Finally, when the stimulus was from a male duet contribution, the male of the pair was most likely to approach the stimulus (Fig. 4
). Finally, when the stimulus was from a male duet contribution, the male of the pair was most likely to approach the stimulus (Fig. 4 ). The number of vocalizations produced in response to the stimuli varied by the type of vocalization (Χ2 = 15.86, df = 6, P = 0.02). Calls (chip and tseet grouped together) were produced in a similar quantity in response to complete duets and each sex’s contributions, and were produced more in response to all conspecific stimuli than to control duets (Fig. 5
). The number of vocalizations produced in response to the stimuli varied by the type of vocalization (Χ2 = 15.86, df = 6, P = 0.02). Calls (chip and tseet grouped together) were produced in a similar quantity in response to complete duets and each sex’s contributions, and were produced more in response to all conspecific stimuli than to control duets (Fig. 5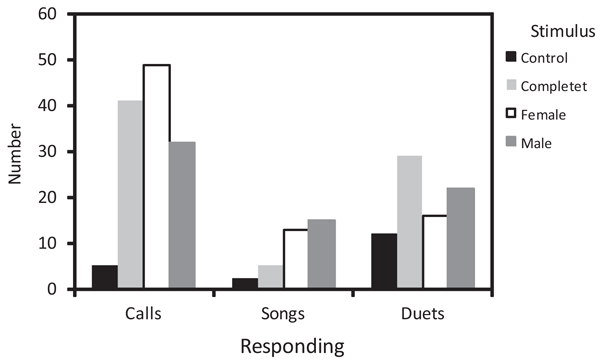 ). Solo songs were produced more in response to male and female duet contributions than to complete and control duets (Fig. 5
). Solo songs were produced more in response to male and female duet contributions than to complete and control duets (Fig. 5 ). Duets were produced more in response to complete duets and male duet contributions than to female duet contributions and control duets (Fig. 5
). Duets were produced more in response to complete duets and male duet contributions than to female duet contributions and control duets (Fig. 5 ).
).
 |
Fig. (5) Comparison of the number of each vocalization type produced by White-eared Ground-sparrow pairs in response to different stimuli used to simulate territorial intrusions. |
4. DISCUSSION
We found a strong approach to complete duets and duet contributions of each sex by territorial pairs of White-eared Ground-sparrows. The characteristics (i.e., frequency, duration, and entropy) of vocalizations that birds produced in response to each type of playback vocalization were different, suggesting that even in the highly overlapped duets of Melozone species [17, 18, 20], duets and each sex’s contribution may have different functions, which is a similar pattern to that found in species where duets are produced from solo songs [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] , 4Hall ML In Naguib M, Janik VM. Ed Advances in the Study of Behavior, 2009; 40: 67-121.].
Both individuals of each territorial pair approached faster and spent more time close to the speaker for all conspecific vocalizations. In our case, the degree of threat is apparently indicated by the number of intruders because playback simulating a duet incited a stronger response than an individual male or female duet contribution. This agrees with the joint resource defense hypothesis because both individuals of the pair responded most aggressively towards an intruder in tandem to jointly defend resources [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] , 4Hall ML In Naguib M, Janik VM. Ed Advances in the Study of Behavior, 2009; 40: 67-121.], which for White-eared Ground-sparrows could be food, territories, or nesting sites [20]. A similar intensity of coordinated approach responses has also been reported in Barred Antshrikes (Thamnophilus doliatus), Tropical Boubous (Laniarius aethiopicus), and Magpie-larks when responding to playback simulating a pair of duetting intruders [19, 26, 27]. These species all produce duets with male and female solo songs [19, 26, 27], contrary to White-eared Ground-sparrows that produce duets with a separate type of duetting vocalization [20, 24]. However, despite the taxonomic differences between species and the manner of producing duets, functions appear to be similar across most species that have been studied [1, 4, 19, 26, 27].
According to the joint resource defense hypothesis, the structure of a vocalization (frequency and time) is expected to change [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] ] because it needs to transmit an aggressive signal to intended receivers. In our case, vocalizations used by birds in response to playback simulating a pair of duetting intruders, such as chip calls, showed a higher frequency of maximum amplitude and maximum frequency, but lower minimum frequency, in responses to complete duets than to individual duet contributions. These characteristics have been associated previously in White-eared Ground-sparrows with higher aggression, because they were the characteristics of vocalizations produced when they defended territories against conspecifics and congeners compared to allopatric closely related species or sympatric non-competitor species [24]. Additionally, in birds and other animals such as frogs, lower minimum frequencies are also associated with more aggressive signals [28 - 30]. Although it has previously been reported that chip call functions in this ground-sparrow, such as contact and mobbing, are encoded in the rate and not in the time and frequency structure of calls [25], production of calls with lower minimum frequency may signal that the pair was more motivated to defend the territory against the intruder [10, 22, 31, 32].
Simulated intrusions with incomplete duets of each sex of White-eared Ground-sparrows showed that pairs responded as predicted by the mate-guarding hypothesis, because each individual within the pair perceived same-sex intruders as more threatening than intruders of the opposite sex [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] , 4Hall ML In Naguib M, Janik VM. Ed Advances in the Study of Behavior, 2009; 40: 67-121.]. Additionally, although duets were produced most often in response to playback of complete duets, they were also produced in response to playback of individual duet contributions. The same chip calls that are used at low rates to communicate within pair members in this ground-sparrow [25] were also produced in response to these types of stimuli, as were tseet calls that are also used to promote duet creation [20, 33]. This suggests that each individual of the pair is trying to create duets with its partner to signal its mating status and prevent its partner from being usurped [34] or leaving the partnership [1, 35]. Both types of responses may indicate a pair’s status and pair bond strength to a same-sex intruder, as has been found in Eastern Whipbirds (Psophodes olivaceus) and Bay Wrens (Cantorchilus nigricapillus) [36-39] when responding to same-sex individual duet contributions.
However, our results also showed that males are using the male solo song, a vocalization also used for mate attraction [20, 24], to respond to individual duet contributions. In this case, the male may use the solo song as an aggressive signal towards male duet contributions to deter rival males from the territory and prevent the partner from being usurped as is predicted by the mate-guarding hypothesis [1Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30.
[http://dx.doi.org/10.1007/s00265-003-0741-x] , 4Hall ML In Naguib M, Janik VM. Ed Advances in the Study of Behavior, 2009; 40: 67-121.]. However, White-eared Ground-sparrow males may use the solo songs in response to female duet contributions as a signal to attract the female and achieve extra-pair copulation [20]. This male behavior may create a conflict because females can deter the male from approaching the female intruder as we observed. This behavior contrasts with other species where duets are produced with male and female solo songs, and females are more willing to sing to create duets and signal pairing status [26, 40]. Our results suggest more clearly than for other species that males have a double agenda for territory intrusions according to the sex of the intruder. If the intruder is a male, their main goal is to defend their mate, but if the intruder is a female, males attempt to attract the intruder and increase the probability of obtaining an extra-pair copulation.
CONCLUSION
In conclusion, White-eared Ground-sparrow territorial pairs use duets in two main ways; first, to protect resources from intruders, supporting the resource defense hypothesis, and second, to protect their partners from being usurped by intruders, supporting the mate-guarding hypothesis. However, our data also showed that males use solo songs both as a signal to attract intruder females and increase their probability of obtaining extra-pair copopulations and to repel intruder males and guard their female mates. In our study species, where mate attraction occurs with a completely different type of vocalization than used for duetting, we found a clear pattern of a double agenda for males when territorial intrusion occurs. Finally, the study of species which use unique vocalizations to produce duets may provide stronger support for the dual function hypothesis, and show more clearly the conflict between individuals of a pair relative to each hypothesis.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This investigation was approved by the scientific committee of Escuela de Biología, Universidad de Costa Rica and with the number N-111- B5-241
HUMAN AND ANIMAL RIGHTS
All research procedures were in accordance with the scientific committee of Escuela de Biología, Universidad de Costa Rica.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Vicerrectoría de Investigación, Universidad de Costa Rica (N-111- B5-241). We thank to Alana Denko and three anonymous reviewers for all valuables comments on the paper draft.
REFERENCES
| [1] | Hall ML. A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 2004; 55: 415-30. [http://dx.doi.org/10.1007/s00265-003-0741-x] |
| [2] | Bailey WJ. Insect duets: Underlying mechanisms and their evolution. Physiol Entomol 2003; 28: 157-74. [http://dx.doi.org/10.1046/j.1365-3032.2003.00337.x] |
| [3] | Burton JA, Nietsch A. Geographic variation in duet songs of Sulawesi tarsiers: Evidence for new cryptic species in south and southeast Sulawesi. Int J Primatol 2010; 31: 1123-46. [http://dx.doi.org/10.1007/s10764-010-9449-8] |
| [4] | Hall ML In Naguib M, Janik VM. Ed Advances in the Study of Behavior, 2009; 40: 67-121. |
| [5] | Benedict L. Occurrence and life history correlates of vocal duetting in North American passerines. J Avian Biol 2008; 39: 57-65. a [http://dx.doi.org/10.1111/j.0908-8857.2008.04103.x] |
| [6] | Price JJ, Lanyon SM, Omland KE. Losses of female song with changes from tropical to temperate breeding in the New World blackbirds. Proc R Soc 2009; 1971-80. [http://dx.doi.org/10.1098/rspb.2008.1626] |
| [7] | Odom KJ, Hall ML, Riebel K, Omland KE, Langmore NE. Female song is widespread and ancestral in songbirds. Nat Commun 2014; 5: 3379. [http://dx.doi.org/10.1038/ncomms4379] [PMID: 24594930] |
| [8] | McDonald DB, Potts WK. Cooperative display and relatedness among males in a lek-mating bird. Science 1994; 266(5187): 1030-2. [http://dx.doi.org/10.1126/science.7973654] [PMID: 7973654] |
| [9] | Maynard DF, Ward KA, Doucet SM, Mennill DJ. Calling in an acoustically competitive environment: Duetting male long-tailed manakins avoid overlapping neighbours but not playback-simulated rivals. Anim Behav 2012; 84: 563-73. [http://dx.doi.org/10.1016/j.anbehav.2012.06.008] |
| [10] | Dahlin CR, Benedict L. Angry birds need not apply: A perspective on the flexible form and multifunctionality of avian vocal duets. Ethology 2014; 120: 1-10. [http://dx.doi.org/10.1111/eth.12182] |
| [11] | Mennill DJ, Vehrencamp SL. Context-dependent functions of avian duets revealed by microphone-array recordings and multispeaker playback. Curr Biol 2008; 18(17): 1314-9. [http://dx.doi.org/10.1016/j.cub.2008.07.073] [PMID: 18771924] |
| [12] | Kahn ZA, Moser-Purdy CE, Mennill DJ. Sing and do not stray: Male Rufous-and-white Wrens use duets and physical behaviours to guard their mates. Anim Behav in press [http://dx.doi.org/10.1016/j.anbehav.2018.07.005] |
| [13] | Hall ML. The function of duetting in magpie-larks: conflict, cooperation, or commitment? Anim Behav 2000; 60(5): 667-77. [http://dx.doi.org/10.1006/anbe.2000.1517] [PMID: 11082237] |
| [14] | Hall ML, Magrath RD. Duetting and mate-guarding in Australian magpie-larks (Grallina cyanoleuca). Behav Ecol Sociobiol 2000; 47: 180-7. [http://dx.doi.org/10.1007/s002650050009] |
| [15] | Benedict L. Unusually high levels of extrapair paternity in a duetting songbird with long-term pair bonds. Behav Ecol Sociobiol 2008; 62: 983-8. b [http://dx.doi.org/10.1007/s00265-007-0524-x] |
| [16] | Benedict L. Context, structural variability and distinctiveness of California towhee (Pipilo crissalis) vocal duets. Ethology 2009; 115: 77-86. [http://dx.doi.org/10.1111/j.1439-0310.2008.01583.x] |
| [17] | Benedict L, McEntee JP. Context, structural variability and distinctiveness of California Towhee (Pipilo crissalis) vocal duets. Ethology 2009; 115: 77-86. [http://dx.doi.org/10.1111/j.1439-0310.2008.01583.x] |
| [18] | Sandoval L, Mennill DJ. A quantitative description of vocalizations and vocal behaviour of Rusty-crowned Ground-sparrow (Melozone kieneri). Ornitol Neotrop 2014; 25: 219-30. |
| [19] | Trejos-Araya C, Barrantes G. Description of the acoustical interaction and synchronization between duetters of the Large-footed Finch (Pezopetes capitalis). Bioacoustics 2018; 27: 183-96. [http://dx.doi.org/10.1080/09524622.2017.1303792] |
| [20] | Sandoval L, Méndez C, Mennill DJ. Vocal behaviour of White-eared Ground-sparrows (Melozone leucotis) during the breeding season: repertoires, diel variation, behaviour context, and individual distinctiveness. J Ornithol 2016; 157: 1-12. [http://dx.doi.org/10.1007/s10336-015-1237-y] |
| [21] | Bolton M. Playback experiments indicate absence of vocal recognition among temporally and geographically separated populations of Madeiran storm-petrels Oceanodroma castro. Ibis 2007; 149: 255-63. [http://dx.doi.org/10.1111/j.1474-919X.2006.00624.x] |
| [22] | Geberzahn N, Goymann W, Muck C, ten Cate C. Females alter their song when challenged in a sex-role reversed bird species. Behav Ecol Sociobiol 2009; 64: 193-204. [http://dx.doi.org/10.1007/s00265-009-0836-0] |
| [23] | Ripmeester EA, Mulder M, Slabbekoorn H. Habitat-dependent acoustic divergence affects playback response in urban and forest populations of the European blackbird. Behav Ecol 2010; 21: 876-83. [http://dx.doi.org/10.1093/beheco/arq075] |
| [24] | Sandoval L, Méndez C, Mennill DJ. Different vocal signals, but not prior experience, influence heterospecific from conspecific discrimination. Anim Behav 2013; 85: 907-15. [http://dx.doi.org/10.1016/j.anbehav.2013.02.006] |
| [25] | Méndez C, Sandoval L. Dual function of chip calls depending on changing call rate related to risk level in territorial pairs of White-eared ground-sparrow. Ethology 2017; 123: 188-96. [http://dx.doi.org/10.1111/eth.12584] |
| [26] | Grafe TU, Bitz JH. Functions of duetting in the tropical boubou, Laniarius aethiopicus: territorial defence and mutual mate guarding. Anim Behav 2004; 68: 193-201. [http://dx.doi.org/10.1016/j.anbehav.2003.11.003] |
| [27] | Koloff J, Mennill DJ. Vocal behaviour of Barred Antshrikes: A Neotropical duetting suboscine songbird. J Ornithol 2013; 154: 51-61. [http://dx.doi.org/10.1007/s10336-012-0867-6] |
| [28] | Davies NB, Halliday TR. Deep croaks and fighting assessment in toads Bufo bufo. Nature 1978; 274: 683-5. [http://dx.doi.org/10.1038/274683a0] |
| [29] | Collins S. Nature’s music - the science of birdsong 2004; 39-78. [http://dx.doi.org/10.1016/B978-012473070-0/50005-0] |
| [30] | Sandoval L, Barrantes G, Ocampo D, Sánchez-Quiros C. Sexual size dimorphism and acoustical features of the pre-advertisement and advertisement calls of Rhinophrynus dorsalis Duméril & Bibron, 1841 (Anura: Rhinophrynidae). Mesoam Herpetol 2015; 2: 154-66. |
| [31] | Morton ES. On the occurrence and significance of motivation-structural rules in some bird and mammal sounds. Am Nat 1977; 111: 855-69. [http://dx.doi.org/10.1086/283219] |
| [32] | Hall ML, Magraht RD. Temporal coordination signals coalition quality. Curr Biol 2007; 17: R406-7. [http://dx.doi.org/10.1016/j.cub.2007.04.022] |
| [33] | Piza P, Sandoval L. The differences in transmission properties of two bird calls show relation to their specific functions. J Acoust Soc Am 2016; 140: 4271-5. [http://dx.doi.org/10.1121/1.4971418] |
| [34] | Appleby BM, Yamaguchi N, Johnson PJ, MacDonald DW. Sex specific territorial responses in tawny owls Strix aluco. Ibis 1999; 141: 91-9. [http://dx.doi.org/10.1111/j.1474-919X.1999.tb04267.x] |
| [35] | Seddon N, Butchart SHM, Odling-Smee L. Duetting in the subdesert mesite Monias benschi: Evidence for acoustic mate defence? Behav Ecol Sociobiol 2002; 52: 7-16. [http://dx.doi.org/10.1007/s00265-002-0488-9] |
| [36] | Levin RN. Song behaviour and reproductive strategies in a duetting wren, Thryothorus nigricapillus: I. Removal experiments. Anim Behav 1996; 52: 1093-106. [http://dx.doi.org/10.1006/anbe.1996.0257] |
| [37] | Mann NI, Marshall-Ball L, Slater PJB. The complex song duet of the plain wren. Condor 2003; 105: 672-82. [http://dx.doi.org/10.1650/7208] |
| [38] | Mann NI, Dingess KA, Barker FK, Graves GR, Slater PJB. A comparative study of song form and duetting in neotropical Thryothorus wrens. Behaviour 2009; 146: 1-43. [http://dx.doi.org/10.1163/156853908X390913] |
| [39] | Rogers AC. Male and female song structure and singing behaviour in the duetting eastern whipbird, Psophodes olivaceus. Aust J Zool 2005; 53: 157-66. [http://dx.doi.org/10.1071/ZO04083] |
| [40] | Seddon N, Tobias JA. Duets defend mates in a suboscine passerine, the warbling antbird (Hypocnemis cantator). Behav Ecol 2006; 17: 73-83. [http://dx.doi.org/10.1093/beheco/ari096] |







