- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Ornithology Journal
(Discontinued)
ISSN: 1874-4532 ― Volume 13, 2020
Glucose Concentrations in Closely Related Titmice (Baeolophus) Species Linked to Regional Habitat Differences Across an Avian Hybrid Zone
Jennifer C. Vaughn1, *, Gary Voelker1, J. Jill Heatley2
Abstract
Aims:
We used physiological data, in conjunction with habitat information, to elucidate the interactions between two hybridizing songbirds within a hybrid zone.
Background:
Hybrid zones are ideal regions to examine a variety of ecological, behavior, and evolutionary processes. In addition to genetics, behavior, and morphology, physiological differences may impact hybrid fitness, genetic introgression, and even the stability of a hybrid zone.
Objective:
To assess physiological differences in hybridizing species, we investigated selected venous blood analytes in two species of songbirds hybridizing along the Balcones Escarpment in central Texas.
Methods:
Using a portable blood analyzer, we assayed blood samples from Black-crested Titmouse (Baeolophus atricristatus) and Tufted Titmouse (B. bicolor) individuals along a longitudinal transect that included the contact zone. Ecologically, this transect varies from higher elevation semi-arid regions on the Balcones Escarpment (and west across the Edwards Plateau) to lower elevation mesic forests east of the escarpment.
Results:
As expected, several blood analytes differed with age, sex, and sedative administration; however, we observed relatively increased blood glucose concentrations in Black-crested Titmice, which occupy the semi-arid habitats of west Texas. Furthermore, glucose concentrations were further elevated following rainfall events. Blood glucose concentrations often increase during stressful conditions and or related to changes in diet.
Conclusion:
We suspect that Black-crested Titmice have relatively increased blood glucose concentrations as a product of living in a semi-arid environment that causes chronic stress from unpredictable food and water resources. The link between rainfall and glucose may be a result of the increased and greater diversity of food availability after rainfall. Although further research is needed, we suspect that habitat differences and associated lack of physiological adaptations may be a limiting factor in westward range expansion in the more aggressive Tufted Titmice.
Article Information
Identifiers and Pagination:
Year: 2020Volume: 13
First Page: 10
Last Page: 23
Publisher Id: TOOENIJ-13-10
DOI: 10.2174/1874453202013010010
Article History:
Received Date: 10/02/2020Revision Received Date: 30/03/2020
Acceptance Date: 15/04/2020
Electronic publication date: 31/07/2020
Collection year: 2020
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Wildlife and Fisheries Sciences and Texas A&M Biodiversity and Research Collections, Texas A&M University, College Station, TX, USA; Tel: +1 214-908-3808; E-mail: jcary@tamu.edu
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 10-02-2020 |
Original Manuscript | Glucose Concentrations in Closely Related Titmice (Baeolophus) Species Linked to Regional Habitat Differences Across an Avian Hybrid Zone | |
1. INTRODUCTION
Geographic regions in which species interbreed or hybridize, commonly known as hybrid zones, are unique locations to examine speciation and adaptation [1Barton NH, Hewitt GM. Analysis of hybrid zones. Annu Rev Ecol Syst 1985; 16: 113-48.
[http://dx.doi.org/10.1146/annurev.es.16.110185.000553] -3Barton NH. The role of hybridization in evolution. Mol Ecol 2001; 10(3): 551-68.
[http://dx.doi.org/10.1046/j.1365-294x.2001.01216.x] [PMID: 11298968] ]. Hybrid zones, in animals, often form via secondary contact of lineages that initially diverged in allopatry [4Swenson NG, Howard DJ. Clustering of contact zones, hybrid zones, and phylogeographic breaks in North America. Am Nat 2005; 166(5): 581-91.
[http://dx.doi.org/10.1086/491688] [PMID: 16224723] ]. Beyond genetics, morphology and behavior are classically often used to assess hybridization, given their importance in species recognition and mate selection [5Miller MJ, Lipshutz SE, Smith NG, Bermingham E. Genetic and phenotypic characterization of a hybrid zone between polyandrous Northern and Wattled Jacanas in Western Panama. BMC Evol Biol 2014; 14(1): 227.
[http://dx.doi.org/10.1186/s12862-014-0227-7] [PMID: 25394718] -8Harrison RG, Larson EL. Hybridization, introgression, and the nature of species boundaries. J Hered 2014; 105(S1)(Suppl. 1): 795-809.
[http://dx.doi.org/10.1093/jhered/esu033] [PMID: 25149255] ]. However, during isolation, sister lineages may also develop physiological adaptations to different environmental conditions [9Eme D, Malard F, Colson-Proch C, et al. Integrating phylogeography, physiology and habitat modelling to explore species range determinants. J Biogeogr 2014; 41(4): 687-99.
[http://dx.doi.org/10.1111/jbi.12237] , 10Kotlik P, Markova S, Vojtek L, Stratil A. Adaptive phylogeography: functional divergence between haemoglobins derived from different glacial refugia in the bank vole. Proceedings of the Royal Society B: Biological Sciences 2014; 281(1786): 20140021.]. Post-isolation, populations and species expansion may be limited or affected by new adaptations. As secondary contact zones usually occur at sharp ecological boundaries (ecotones), the impact of physiology on distribution may be a critical component to understanding species or populations interactions, especially hybridization of species [2Abbott R, Albach D, Ansell S, et al. Hybridization and speciation. J Evol Biol 2013; 26(2): 229-46.
[http://dx.doi.org/10.1111/j.1420-9101.2012.02599.x] [PMID: 23323997] , 11Endler JA. Geographic Variation, Speciation, and Clines 1977.].
Physiology is known to play a role in limiting geographic range, but some hybrid zone studies have supported physiological differences as limiting factors to distribution [12Bozinovic F, Naya DE. Linking Physiology, Climate, and Species Distributional Ranges.Integrative Organismal Biology 1st ed. 2015; 14.]. Once range expansion or distribution is limited, genetic introgression, selection, and other evolutionary forces are impacted. For example, spatial distribution in two hybridizing swordtail fish was linked to differences in heat-tolerant gene expression in varying water temperatures [13Culumber ZW, Shepard DB, Coleman SW, Rosenthal GG, Tobler M. Physiological adaptation along environmental gradients and replicated hybrid zone structure in swordtails (Teleostei: Xiphophorus). J Evol Biol 2012; 25(9): 1800-14.
[http://dx.doi.org/10.1111/j.1420-9101.2012.02562.x] [PMID: 22827312] ]. In birds, hybrid tit-tyrant flycatchers in the Andes mountains are not found at the higher elevations preferred by one parental species because they are not physiologically adapted to high elevation living [14DuBay SG, Witt CC. Differential high-altitude adaptation and restricted gene flow across a mid-elevation hybrid zone in Andean tit-tyrant flycatchers. Mol Ecol 2014; 23(14): 3551-65.
[http://dx.doi.org/10.1111/mec.12836] [PMID: 24943893] ]. Thus, scientists must consider physiological adaptations in hybridization research.
In this study, we investigated physiological differences between two species of hybridizing songbirds in central Texas. Based on similar studies and new evidence, we suspect that differences are linked to regional habitats and/or climatic distinctions on either side of the hybrid zone.
1.1. Texas Hybrid Zone
In Texas, the Balcones Escarpment (BE) is a southwest-to-northcentral oriented inactive fault line that creates a strong west to east ecological boundary [15Cadenasso ML, Pickett STA, Weathers KC, Jones CG. A framework for a theory of ecological boundaries. Bioscience 2003; 53(8): 750-8.
[http://dx.doi.org/10.1641/0006-3568(2003)053[0750:AFFATO]2.0.CO;2] , 16Abbott P, Woodruff CM Jr. The balcones escarpment: Geology, hydrology, ecology, and social development in Central Texas 1986.]. West of the BE, the Edwards Plateau ranges in elevation from 180 m to nearly 900 m with a semi-arid climate dominated by juniper-oak vegetation, receiving an average of 380-860 mm of rainfall per year, with the majority occurring in May or June [17Griffith G, Bryce S, Omernick J, Rogers A. Cartographers. Ecoregions of Texas 2007.]. East of the BE, lower elevation (0-180 m) and a more temperate climate characterize the more mesic habitats of the Blackland Prairie and Post-Oak forests nearer the BE, and the Piney Woods of the interior coastal plains in eastern-most Texas [17Griffith G, Bryce S, Omernick J, Rogers A. Cartographers. Ecoregions of Texas 2007., 18Webb WL. Biogeographic regions of Texas and Oklahoma. Ecology 1950; 31(3): 426-33.
[http://dx.doi.org/10.2307/1931496] ]. Rainfall in these ecoregions is fairly evenly distributed annually with an average of 700-1000 mm in the Post-Oak and Blackland Prairie regions and 900-1300 mm in the Piney Woods [17Griffith G, Bryce S, Omernick J, Rogers A. Cartographers. Ecoregions of Texas 2007., 18Webb WL. Biogeographic regions of Texas and Oklahoma. Ecology 1950; 31(3): 426-33.
[http://dx.doi.org/10.2307/1931496] ].
With this change in elevation, vegetation and rainfall regimes on and east of the BE coincide with a longitudinal distributional barrier for numerous vertebrate taxa, including birds [19Goetze JR. Distribution, natural history, and biogeographic relationships of mammals on the Edwards Plateau of Texas 1995.-21Owen JG, Dixon JR. An ecogeographic analysis of the herpetofauna of Texas. Southwest Nat 1989; 34(2): 165-80.
[http://dx.doi.org/10.2307/3671726] ]. In addition to distributional range limits occurring at the escarpment, some species-pairs are thought to hybridize at this ecological interface [22Smith HM, Buechner HK. The influence of the Balcones Escarpment on the distribution of amphibians and reptiles in Texas. Bullentin of the Chicago Academy of Sciences 1947; 8(1): 1-16., 23Dixon KL. Contact zones of avian congeners on the southern Great Plains. Condor 1989; 91(1): 15-22.
[http://dx.doi.org/10.2307/1368143] ]. Avian species-pair recently confirmed to be hybridizing at this interface are the Black-crested Titmouse (Baeolophus atricristatus) and Tufted Titmouse (B. bicolor) [23Dixon KL. Contact zones of avian congeners on the southern Great Plains. Condor 1989; 91(1): 15-22.
[http://dx.doi.org/10.2307/1368143] ]. Black-crested Titmice are primarily found in the more arid-scrubby habitat to the west and south (to include northern Mexico) of the BE ecotone but are occasionally found east of the BE [24Patten MA, Smith-Patten BD. Black-crested Titmouse (Baeolophus atricristatus). [Internet]. Cornell Lab of Ornithology 2008. Available from:
http://bna.birds.cornell.edu.lib-ezproxy.tamu.edu:2048/bna/
species/717/articles/introduction, 25Ritchison G, Grubb TC Jr, Pravosudov VV. Tufted Titmouse (Baeolophus bicolor). [Internet]. Cornell Lab of Ornithology 2015. Available from:
http://bna.birds.cornell.edu/bna/species/086/articles
/introduction]. Tufted Titmice are found in the more mesic, deciduous forests east of the BE and throughout the eastern United States and Canada [25Ritchison G, Grubb TC Jr, Pravosudov VV. Tufted Titmouse (Baeolophus bicolor). [Internet]. Cornell Lab of Ornithology 2015. Available from:
http://bna.birds.cornell.edu/bna/species/086/articles
/introduction, 26Dixon KL. An ecological analysis of the inter-breeding of crested titmice in Texas. Univ Calif Publ Zool 1955; 54(3): 125-206.].
While these non-migrating sister species share similar diet, breeding/nesting behavior, and general patterns of intraspecific communication, they differ in plumage, song, morphology and habitat preference [24Patten MA, Smith-Patten BD. Black-crested Titmouse (Baeolophus atricristatus). [Internet]. Cornell Lab of Ornithology 2008. Available from:
http://bna.birds.cornell.edu.lib-ezproxy.tamu.edu:2048/bna/
species/717/articles/introduction, 25Ritchison G, Grubb TC Jr, Pravosudov VV. Tufted Titmouse (Baeolophus bicolor). [Internet]. Cornell Lab of Ornithology 2015. Available from:
http://bna.birds.cornell.edu/bna/species/086/articles
/introduction]. Currently ranked as separate species, their taxonomic status has fluctuated between species and subspecies because ornithologists have postulated hybridization [24Patten MA, Smith-Patten BD. Black-crested Titmouse (Baeolophus atricristatus). [Internet]. Cornell Lab of Ornithology 2008. Available from:
http://bna.birds.cornell.edu.lib-ezproxy.tamu.edu:2048/bna/
species/717/articles/introduction, 25Ritchison G, Grubb TC Jr, Pravosudov VV. Tufted Titmouse (Baeolophus bicolor). [Internet]. Cornell Lab of Ornithology 2015. Available from:
http://bna.birds.cornell.edu/bna/species/086/articles
/introduction]. Using morphological measurements and crest plumage, Dixon identified several hybrid zones, with the primary one located in central Texas [26Dixon KL. An ecological analysis of the inter-breeding of crested titmice in Texas. Univ Calif Publ Zool 1955; 54(3): 125-206.]. He estimated this zone to be approximately 50 km wide and 175 km long (Fig. 1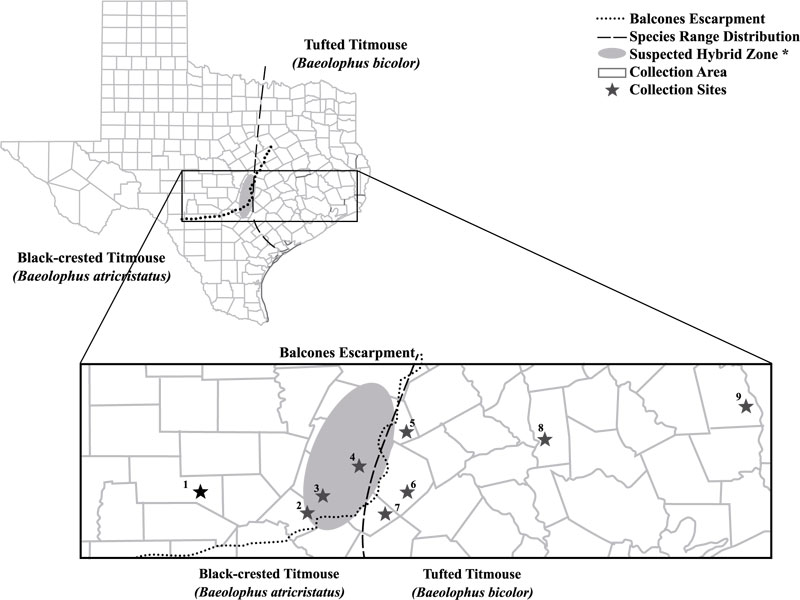 ) [23Dixon KL. Contact zones of avian congeners on the southern Great Plains. Condor 1989; 91(1): 15-22.
) [23Dixon KL. Contact zones of avian congeners on the southern Great Plains. Condor 1989; 91(1): 15-22.
[http://dx.doi.org/10.2307/1368143] , 26Dixon KL. An ecological analysis of the inter-breeding of crested titmice in Texas. Univ Calif Publ Zool 1955; 54(3): 125-206.]. Curry and Patten recently investigated plumage and morphological variation within this central hybrid zone and their findings support Dixon’s assessments of hybridization [27Curry CM, Patten MA. Current and historical extent of phenotypic variation in the Tufted and Black-crested titmouse (Paridae) hybrid zone in the southern Great Plains. Am Midl Nat 2014; 171(2): 271-300.
[http://dx.doi.org/10.1674/0003-0031-171.2.271] , 28Curry CM, Patten MA. Shadow of a doubt: premating and postmating isolating barriers in a temporally complex songbird (Passeriformes: Paridae) hybrid zone. Behav Ecol Sociobiol 2016; 70(8): 1171-86.
[http://dx.doi.org/10.1007/s00265-016-2126-y] ]. In a related study, Vaughn and Voelker (in progress) provide genetic evidence of hybridization in birds captured near the BE.
Since this hybrid zone occurs at a strong ecotone, we aim to investigate which blood gases and electrolytes differ between Tufted and Black-crested Titmice and assess if the differences are linked to climate and/or habitat. In a previous study of a suite of passerines, including titmice, we observed differences in venous blood analytes between individuals sampled east of the BE, relative to those sampled west of the BE [29Heatley JJ, Cary J, Kingsley L, Beaufrere H, Russell KE, Voelker G. Midazolam sedates Passeriformes for field sampling but affects multiple venous blood analytes. Vet Med (Auckl) 2015; 6: 61-9.
[http://dx.doi.org/10.2147/VMRR.S71402] [PMID: 30155435] , 30Heatley JJ, Cary J, Russell KE, Voelker G. Clinicopathological analysis of Passeriform venous blood reflects tranitions in elevation and habitat. Veterinary Medicine: Research and Reports 2013; 4: 21-9.]. We hypothesize that, between species, differences in blood analyte concentrations will be strongly correlated with habitat or climatic differences associated with the different ecoregions east and west of the BE. We also aim to report the role that other non-habitat variables (age, sex, and methodology) have on blood gas and electrolytes within two closely related species. The aim is to expand the knowledge of the variability of blood gas and electrolytes in wild passerines as well as provide a better understanding of ecological impacts on physiological differences between closely related, hybridizing species.
2. MATERIALS AND METHODS
2.1. Capture and Sampling
From March-August in 2010-2012, we captured 54 titmice (Baeolophus spp.) on private properties in central Texas (Fig. 1 ). Most birds were collected via mist nests from 0630-1200 CST; two were caught between 1700-2000 CST. We collected 26 titmice west of the Balcones Escarpment; two of these were captured west of the contact zone (Table 1) [26Dixon KL. An ecological analysis of the inter-breeding of crested titmice in Texas. Univ Calif Publ Zool 1955; 54(3): 125-206., 27Curry CM, Patten MA. Current and historical extent of phenotypic variation in the Tufted and Black-crested titmouse (Paridae) hybrid zone in the southern Great Plains. Am Midl Nat 2014; 171(2): 271-300.
). Most birds were collected via mist nests from 0630-1200 CST; two were caught between 1700-2000 CST. We collected 26 titmice west of the Balcones Escarpment; two of these were captured west of the contact zone (Table 1) [26Dixon KL. An ecological analysis of the inter-breeding of crested titmice in Texas. Univ Calif Publ Zool 1955; 54(3): 125-206., 27Curry CM, Patten MA. Current and historical extent of phenotypic variation in the Tufted and Black-crested titmouse (Paridae) hybrid zone in the southern Great Plains. Am Midl Nat 2014; 171(2): 271-300.
[http://dx.doi.org/10.1674/0003-0031-171.2.271] ]. East of the BE, we collected 28 titmice. In a related project, we determined that plumage is not a reliable indicator of hybridization. Therefore, for this study, we used mitochondrial DNA for species assignment for all individuals in this study.
After removal from the mist net, titmice were held in cloth bags for 5-15 min before blood collection. As part of a larger study, Titmice captured in 2012 (n = 26) were administered 0.01-0.03 mL midazolam (3.9 +/-1.8 mg kg-1; 5mg ml-1 Injection USP Hospira, Inc.) intra-nasally prior to blood collection for sedation [29Heatley JJ, Cary J, Kingsley L, Beaufrere H, Russell KE, Voelker G. Midazolam sedates Passeriformes for field sampling but affects multiple venous blood analytes. Vet Med (Auckl) 2015; 6: 61-9.
[http://dx.doi.org/10.2147/VMRR.S71402] [PMID: 30155435] ]. Midazolam is a safe and effective sedative of wild birds [31Mans C, Guzman DSM, Lahner LL, Paul-Murphy J, Sladky KK. Sedation and physiologic response to manual restraint after intranasal administration of midazolam in Hispaniolan Amazon parrots (Amazona ventralis). J Avian Med Surg 2012; 26(3): 130-9.
[http://dx.doi.org/10.1647/2011-037R.1] [PMID: 23156974] -33Schaffer DPH, de Araújo NLLC, Raposo ACS, Filho EFM, Vieira JVR, Oriá AP. Sedative effects of intranasal midazolam administration in wild caught Blue-fronted Amazon (Amazona aestiva) and Orange-winged Amazon (Amazona amazonica) Parrots. J Avian Med Surg 2017; 31(3): 213-8.
[http://dx.doi.org/10.1647/2016-201] [PMID: 28891701] ]. Clinical effects of sedation were observed and recorded on a sedation scale [29Heatley JJ, Cary J, Kingsley L, Beaufrere H, Russell KE, Voelker G. Midazolam sedates Passeriformes for field sampling but affects multiple venous blood analytes. Vet Med (Auckl) 2015; 6: 61-9.
[http://dx.doi.org/10.2147/VMRR.S71402] [PMID: 30155435] ]. Following rest or sedation plus rest, we obtained blood samples (200-500 µL) via jugular venipuncture and immediately transferred to lithium heparin Microtainer tubes (Sarstedt Inc., USA) for anticoagulation prior to analysis.
 |
Fig. (1) Map of locations for sampling (square) of Tufted and Black-crested Titmice. Counties west of the Balcones Escarpment (dashed line) include Kerr (1), Comal (2), Hays (3), and Travis (4). Counties east of the Escarpment are Williamson (5), Bastrop (6), Caldwell (7), Grimes (8), and Tyler (9). The extent of Titmouse species’ distribution range in Texas (dotted line) and central contact zone [26Dixon KL. An ecological analysis of the inter-breeding of crested titmice in Texas. Univ Calif Publ Zool 1955; 54(3): 125-206.] (shaded oval) are also shown. Map modified from EnchantedLearning.com (2018). |
Samples were analyzed in the field using an i-STAT 1 system (Abbott Laboratories, USA), following standard field protocols [30Heatley JJ, Cary J, Russell KE, Voelker G. Clinicopathological analysis of Passeriform venous blood reflects tranitions in elevation and habitat. Veterinary Medicine: Research and Reports 2013; 4: 21-9.]. Cartridges that evaluated blood gases were used first to minimize the change in analytes based on time-lapse from sampling [29Heatley JJ, Cary J, Kingsley L, Beaufrere H, Russell KE, Voelker G. Midazolam sedates Passeriformes for field sampling but affects multiple venous blood analytes. Vet Med (Auckl) 2015; 6: 61-9.
[http://dx.doi.org/10.2147/VMRR.S71402] [PMID: 30155435] ]. Remaining cartridges were analyzed within 5 min of blood collection. Venous blood analytes measured by the i-STAT 1 system were pH, carbon dioxide partial pressure (pCO2), oxygen partial pressure (pO2), lactate, ionized calcium (iCa+), glucose, sodium (Na+), potassium (K+), chloride (Cl-), blood urea nitrogen (BUN), and hematocrit (Hct). The i-STAT 1 system also determined, via calculations, bicarbonate (HCO3), total carbon dioxide (tCO2), base excess (BEecf), dissolved venous oxygen (sO2), and hemoglobin (Hb) [34Abbott Point of Care i-STAT System Manual: Hematocrit/HCT and calculated hemogloblin/HB 2016., 35Abbott Point of Care i-STAT System Manual: PCO2 and calculated values for HCO3, base excess and anion gap 2017.]. Packed red blood cell volume (PCV) was also determined via standard centrifugal methods [30Heatley JJ, Cary J, Russell KE, Voelker G. Clinicopathological analysis of Passeriform venous blood reflects tranitions in elevation and habitat. Veterinary Medicine: Research and Reports 2013; 4: 21-9.].
Following sampling, we humanely euthanized and deposited birds as museum specimens at the Biodiversity Research and Teaching Collections (BRTC) at Texas A&M University (College Station, TX). Morphological and genetic information from these birds will be used in future studies. This research was conducted under required Texas Parks and Wildlife (permit# SPR-0209-016) and United States Fish and Wildlife Permits (permit #188 MB205752) and with approval of the Texas A&M University Institutional Animal Care and Use Committee (AUP# 2012-6).
2.2. Statistical Analysis
All venous blood analytes (n = 16) were assessed for normality using a Shapiro-Wilk Goodness of Fit Test (α = 0.05). We transformed analytes, which were not normally distributed and tested for, and removed, any outliers. Thus, for all subsequent analyses, we used the log of lactate and tCO2, the reciprocal of Hct, and the reciprocal of the exponent of pH. As obtaining the reciprocal square root failed to normalize BUN data, we categorized this analyte as less than 3 mg/dL or greater or equal to 3 mg/dL or greater. We performed non- parametric tests for further statistical analysis of this analyte.
We further explored possible confounding factors. We tested all analytes against age (juvenile, adult), sex (male, female), and administration of midazolam (yes, no) as well as differences between populations based upon capture locality (west, contact zone, east) and location relative to BE (east, west) (Fig. 1 , Table 1). For each analyte and factor, heteroscedasticity was tested using a 2-sided, 2 factor F Test and the 3 factor Bartlett test. If variances were unequal, as in Hct, we utilized Welch’s test to assess significant differences between means, and we weighted models to account for heteroscedasticity. For variables with equal variance, we used a one-way analysis of variance (ANOVA) tests and paired T-tests to compare population differences and the effect of sample size (α = 0.05).
, Table 1). For each analyte and factor, heteroscedasticity was tested using a 2-sided, 2 factor F Test and the 3 factor Bartlett test. If variances were unequal, as in Hct, we utilized Welch’s test to assess significant differences between means, and we weighted models to account for heteroscedasticity. For variables with equal variance, we used a one-way analysis of variance (ANOVA) tests and paired T-tests to compare population differences and the effect of sample size (α = 0.05).
We further tested related or similar variables using correlation analysis and Bland Altman plots to assess interactions between multiple variables for those analytes showing significance. The i-STAT 1 system calculates sO2 from measurements of pO2, pH, and HCO3. The matched-paired analysis confirmed a correlation of sO2 and pO2, as well as sO2 with pH and HCO3 [35Abbott Point of Care i-STAT System Manual: PCO2 and calculated values for HCO3, base excess and anion gap 2017.]. Therefore, we opted not to analyze sO2 in future analyses and focus on the measured value of pO2. The i-STAT 1 system calculates hemoglobin by using the measured hematocrit and multiplying by a factor of 0.34, creating a manufactured value [34Abbott Point of Care i-STAT System Manual: Hematocrit/HCT and calculated hemogloblin/HB 2016.]. We confirmed the correlation between Hct and Hgb (0.925, p <0.001) and then focused on Hct differences across variables. Hematocrit (Hct) from the i-STAT 1 system and PCV determined via centrifuge were compared via the Tukey Mean Difference plot. We incorporated rainfall data and geographic location in two-way ANOVAs or linear regressions to determine if there was a biological reason for those analytes showing statistical significance. All values in the results section are means with standard error, unless reported otherwise. All significance tests set alpha at 0.05.
3. RESULTS
3.1. Sample Collection
We analyzed venous blood samples from 54 titmice (Baeolophus spp.) (22 juveniles, 33 adults) (Table 1). In the field, using plumage, 27 were identified as Black-crested Titmice (Baeolophus atricristatus), 23 as Tufted Titmice (B. bicolor), and four as hybrids. Mitochondrial DNA, from a related genetic study, identified the four phenotypic hybrids as one Black-crested and three Tufted Titmice (Vaughn and Voelker, in progress). Two phenotypic Black-crested Titmice captured east of the BE (Grimes and Williamson counties) actually classified as Tufted Titmice from mtDNA [24Patten MA, Smith-Patten BD. Black-crested Titmouse (Baeolophus atricristatus). [Internet]. Cornell Lab of Ornithology 2008. Available from: http://bna.birds.cornell.edu.lib-ezproxy.tamu.edu:2048/bna/ species/717/articles/introduction].
3.2. Age, Sex, and Midazolam
Sex and age of titmice affected relatively few analytes. Females had increased potassium concentrations compared to males (ANOVA, F2 = 4.77, p = 0.03, Table 2). Hematocrit, as measured with an i-STAT 1 system (Hct), failed to agree with the traditional centrifuge method (PCV) (Tukey Mean Difference -12.6, SE 0.9, p <0.001) (Table 2). We confirmed the correlation of Hct and Hb (0.93, p <0.001) and thus focused on Hct differences based on age and sex. Hematocrit (Hct) was higher in adult titmice compared to juveniles (Transformed 1/Hct, Welch’s Test, t1 = 2.03, p = 0.05) and concentrations of hemoglobin (Hb) showed a similar trend (Welch’s Test, t1 = 1.97, p = 0.05) (means, SE in Table 2).
Midazolam administration affected all blood analytes except pH, Hct, PCV, and Hb (Table 3). Midazolam administration was associated with increased values of pCO2, bicarbonate, tCO2, base excess, glucose, and sodium but lower values of pO2, sO2, lactate, potassium, chloride, and blood urea nitrogen. Midazolam administration was associated with relatively decreased pO2 in juveniles compared with adults receiving midazolam, while pO2 was comparably lower in adults than juveniles, regardless of the administration of midazolam (ANOVA, F3 = 6.47, p <0.001), (Table 4, Fig. 2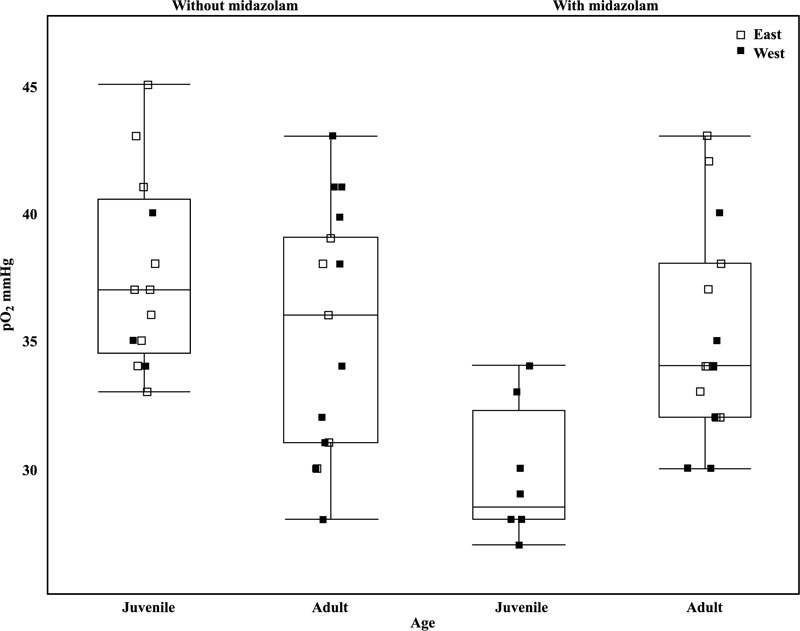 ).
).
3.3. Population and Balcones Escarpment
Blood glucose concentrations of titmice differed significantly based on population (west, contact zone, east) (Table 3). Titmice captured within the contact zone had the highest glucose concentrations, followed by western and then eastern populations (Table 3). Administration of the sedative midazolam also increased glucose concentrations, but the location of capture was a stronger influence of glucose than sedative (east vs. west of BE) (2-way ANOVA, Table 4).
Blood glucose concentrations were related to relative rainfall (higher or lower) and “days since rainfall” (Table 4). For titmice captured west of the BE, blood glucose concentrations were increased in months of high rainfall. However, for birds captured east of the BE, no difference of blood glucose was noted in association with levels of monthly rainfall (Fig. 3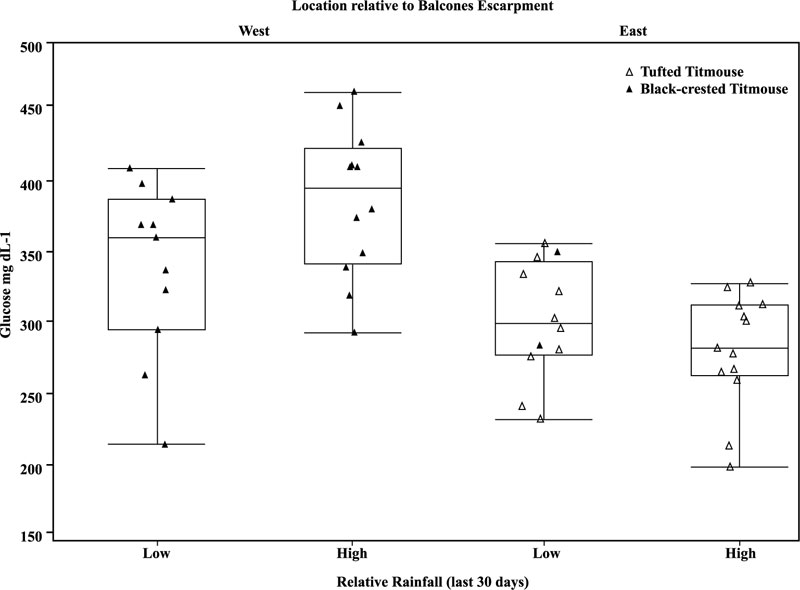 ). In titmice captured west of the BE, length of time since rainfall was positively correlated with higher blood glucose concentrations occurring after 12-25 days after rainfall (Linear Regression R2 = 0.40, ANOVA, F3 = 9.83, p <0.001) (Table 4, Fig. 4
). In titmice captured west of the BE, length of time since rainfall was positively correlated with higher blood glucose concentrations occurring after 12-25 days after rainfall (Linear Regression R2 = 0.40, ANOVA, F3 = 9.83, p <0.001) (Table 4, Fig. 4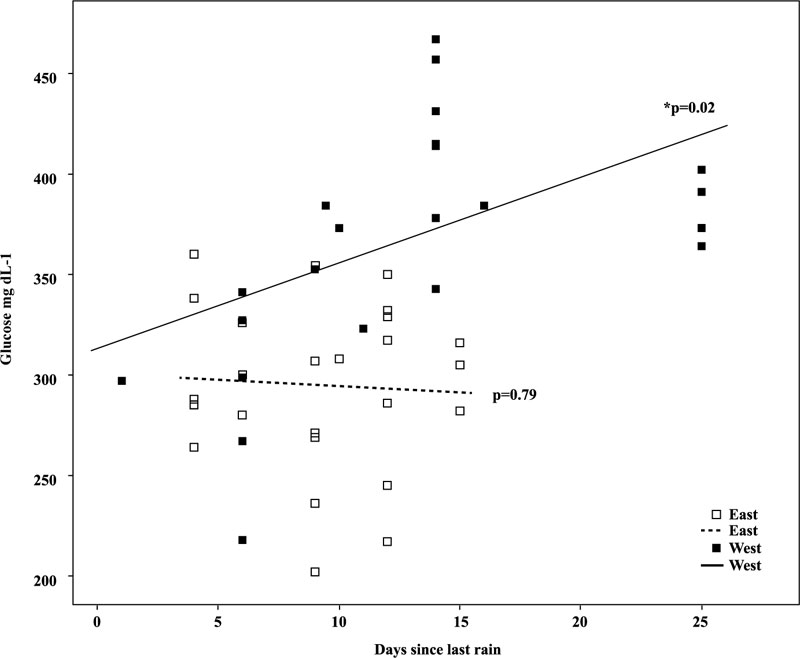 ).
).
Other venous blood analytes that varied significantly based on population, primarily between the contact zone and eastern populations included pO2, sO2, pH and HCO3. Both pO2 and sO2 values were significantly lower in individuals within the contact zone compared to the eastern population (Table 3). Although the western population had the highest values for pO2 and sO2, these did not differ significantly from contact zone or eastern population values (Table 3). Midazolam administration resulted in decreased pO2 values, with a more pronounced effect in Titmice west of the BE (ANOVA, F1 = 5.10, p =0.03, Table 3). No interaction of capture location and midazolam administration was apparent (ANOVA, t2 = -0.99, p = 0.33, Table 4).
4. DISCUSSION
Venous blood glucose concentrations of titmice differed east and west of the BE and were also relatively increased in birds captured west of the BE, especially during months with higher rainfall.
4.1. Location and Rainfall Increase Venous Blood Glucose Concentrations
Of 16 blood venous blood analytes assessed in two parapatric species of titmice, only blood glucose concentrations were statistically significantly different east and west of the BE, with relatively increased values west of the BE. A small sample size from the western population and inherent analyte variability likely prevented statistical distinctions between the western and hybrid zone populations (Table 3). The difference in glucose east and west of the BE was also observed in our related studies of multiple Texas passerine species [29Heatley JJ, Cary J, Kingsley L, Beaufrere H, Russell KE, Voelker G. Midazolam sedates Passeriformes for field sampling but affects multiple venous blood analytes. Vet Med (Auckl) 2015; 6: 61-9.
[http://dx.doi.org/10.2147/VMRR.S71402] [PMID: 30155435] , 30Heatley JJ, Cary J, Russell KE, Voelker G. Clinicopathological analysis of Passeriform venous blood reflects tranitions in elevation and habitat. Veterinary Medicine: Research and Reports 2013; 4: 21-9.]. Although pO2 and sO2 also differed between titmice east and west of the BE, differences resulted from age, sex, and/or midazolam administration.
In general, within bird species, glucose can vary by species, age, time of year, or can be elevated by physiological or psychological stress [36Lill A. Sources of variation in blood glucose concentrations of free-living birds. Avian Biol Res 2011; 4(2): 78-86.
[http://dx.doi.org/10.3184/175815511X13073729328092] -39Kaliński A, Bańbura M, Glądalski M, et al. Long-term variation in blood glucose concentration in nestling Great Tits (Parus major). Avian Biol Res 2015; 8(3): 129-37.
[http://dx.doi.org/10.3184/175815515X14294426911072] ]. In birds, blood glucose concentrations are modulated by Glucocorticoid (GC) production pathways via two different receptors. Baseline GC production is mediated by the mineralocorticoid receptor from which increased production can occur following an increase in energetic demands, thermoregulatory changes, or even a period of low food abundance [37Tomasek O, Bobek L, Kralova T, Adamkova M, Albrecht T. Fuel for the pace of life: Baseline blood glucose concentration co-evolves with life-history traits in songbirds. Funct Ecol 2019; 33(2): 239-49.
[http://dx.doi.org/10.1111/1365-2435.13238] -40Hau M, Casagrande S, Ouyang JQ, Baugh AT. Chapter two - glucocorticoid-mediated phenotypes in vertebrates: Multilevel variation and evolution advances in the study of behavior 48 2016; 41-115.]. Stress-induced GC production, however, is mediated through glucocorticoid receptors that upregulate gluconeogenesis based on 2-10 fold higher concentration of glucocorticoid than baseline.
 |
Fig. (3) Venous blood glucose concentrations (mg dL-1) of Titmice captured west and east of the Balcones Escarpment (BE) based on relative monthly rainfall data. Black-crested Titmice are represented by solid triangles and Tufted Titmice represented with open triangles. Titmice captured west of the BE had relatively increased concentrations of glucose than those captured east (R2=0.33, p<0.001). Concentrations were further elevated west of the escarpment during months of relatively high rainfall (BE p<0.001, relative rainfall p = 0.34, BE*relative rainfall p = 0.03; whole model R2 = 0.38, p<0.001; Table 4). |
Titmice captured west of the Balcones Escarpment have relatively increased glucose values compared with that east of the BE (Tufted Titmice), irrespective of sedative administration. Black-crested and Tufted Titmice share many life history similarities but differ in their habitat preference [24Patten MA, Smith-Patten BD. Black-crested Titmouse (Baeolophus atricristatus). [Internet]. Cornell Lab of Ornithology 2008. Available from:
http://bna.birds.cornell.edu.lib-ezproxy.tamu.edu:2048/bna/
species/717/articles/introduction, 25Ritchison G, Grubb TC Jr, Pravosudov VV. Tufted Titmouse (Baeolophus bicolor). [Internet]. Cornell Lab of Ornithology 2015. Available from:
http://bna.birds.cornell.edu/bna/species/086/articles
/introduction]. Black-crested resides in the semi-arid scrub habitat of west Texas where precipitation is more sporadic, a factor that can lead to unpredictable food resources and induce physiological stress [17Griffith G, Bryce S, Omernick J, Rogers A. Cartographers. Ecoregions of Texas 2007., 41Nielsen-Gammon JW. Changing climate of texas. Impact of global warming on texas 2nd ed. 2011; 39-68.-43Yang LH, Bastow JL, Spence KO, Wright AN. What can we learn from resource pulses? Ecology 2008; 89(3): 621-34.
[http://dx.doi.org/10.1890/07-0175.1] [PMID: 18459327] ]. Low food abundance can increase baseline glucose concentrations. Survival and adaptation to a continually altered physiological state, due to constant stress, is known as the Chronic Stress Hypothesis [44Cherel Y, Robin J-P, Maho YL. Physiology and biochemistry of long-term fasting in birds. Can J Zool 1988; 66(1): 159-66.
[http://dx.doi.org/10.1139/z88-022] -46Fokidis HB, des Roziers MB, Sparr R, Rogowski C, Sweazea K, Deviche P. Unpredictable food availability induces metabolic and hormonal changes independent of food intake in a sedentary songbird. J Exp Biol 2012; 215(Pt 16): 2920-30.
[http://dx.doi.org/10.1242/jeb.071043] [PMID: 22837467] ]. In an arid habitat, chronic stress may be due to scarce or unpredictable food or water sources and, over the long term, such stresses can induce adaptations to increase survivability [44Cherel Y, Robin J-P, Maho YL. Physiology and biochemistry of long-term fasting in birds. Can J Zool 1988; 66(1): 159-66.
[http://dx.doi.org/10.1139/z88-022] , 46Fokidis HB, des Roziers MB, Sparr R, Rogowski C, Sweazea K, Deviche P. Unpredictable food availability induces metabolic and hormonal changes independent of food intake in a sedentary songbird. J Exp Biol 2012; 215(Pt 16): 2920-30.
[http://dx.doi.org/10.1242/jeb.071043] [PMID: 22837467] , 47Williams J, Tieleman BI. Physiological ecology and behavior of desert birds.Current Ornithology Current Ornithology 16 2001; 299-353.
[http://dx.doi.org/10.1007/978-1-4615-1211-0_6] ].
Evidence for a an increased natural glucose concentration based on intermittent food/water resources is shown by the further increase in Black-crested glucose concentration following rain events, a pattern not observed in Tufted Titmice. In arid and semi-arid regions, rainfall amplifies primary productivity and triggers an increase in insect and fruit abundance, stimulating foraging by secondary and tertiary consumers [43Yang LH, Bastow JL, Spence KO, Wright AN. What can we learn from resource pulses? Ecology 2008; 89(3): 621-34.
[http://dx.doi.org/10.1890/07-0175.1] [PMID: 18459327] , 48Ostfeld RS, Keesing F. Pulsed resources and community dynamics of consumers in terrestrial ecosystems. Trends Ecol Evol (Amst) 2000; 15(6): 232-7.
[http://dx.doi.org/10.1016/S0169-5347(00)01862-0] [PMID: 10802548] , 49Wenninger EJ, Inouye RS. Insect community response to plant diversity and productivity in a sagebrush–steppe ecosystem. J Arid Environ 2008; 72(1): 24-33.
[http://dx.doi.org/10.1016/j.jaridenv.2007.04.005] ]. We suspect that sporadic rain showers temporarily increase food resources, causing hyperphagia or an increase in metabolic activity (a known correlate of glucose concentrations). A rise in blood glucose has been observed in organisms exposed to unpredictable or pulse food resources as a result of a change in diet (e.g., sugar-filled berries) or a product of hyperphagia [46Fokidis HB, des Roziers MB, Sparr R, Rogowski C, Sweazea K, Deviche P. Unpredictable food availability induces metabolic and hormonal changes independent of food intake in a sedentary songbird. J Exp Biol 2012; 215(Pt 16): 2920-30.
[http://dx.doi.org/10.1242/jeb.071043] [PMID: 22837467] ]. We observed blood glucose concentrations were highest in birds captured 12-25 days following rain, likely a result of the time lag between rainfall and pulse food resources from increased primary productivity [38Jimeno B, Hau M, Verhulst S. Corticosterone levels reflect variation in metabolic rate, independent of ‘stress’. Sci Rep 2018; 8(1): 13020.
[http://dx.doi.org/10.1038/s41598-018-31258-z] [PMID: 30158537] , 47Williams J, Tieleman BI. Physiological ecology and behavior of desert birds.Current Ornithology Current Ornithology 16 2001; 299-353.
[http://dx.doi.org/10.1007/978-1-4615-1211-0_6] , 48Ostfeld RS, Keesing F. Pulsed resources and community dynamics of consumers in terrestrial ecosystems. Trends Ecol Evol (Amst) 2000; 15(6): 232-7.
[http://dx.doi.org/10.1016/S0169-5347(00)01862-0] [PMID: 10802548] ]. Glucose concentrations in Tufted Titmice, on the other hand, did not increase following rain events, lending support to the idea that these species, living in mesic habitats, have a continual food source year round and, thus, are not prone to infrequent bouts of low food sources. It is interesting to note that the two phenotypically (plumage) Black-crested Titmice captured east of the BE did not express similar glucose concentrations to those captured west. The adult male Black-crested Titmouse (Williamson county) had a glucose concentrations slightly less than the average of birds west of the BE (354 mg/dL, x = 367 mg/dL), but the juvenile male Black-crested (Grimes county) had a glucose value less than the average of birds east of the BE (288 mg/dL, x = 294 mg/dL). Both birds received a sedative and were captured in late spring; however, the adult male was captured just east of the BE (closer to the hybrid zone). Therefore, though these birds look Black-crested and have Tufted Titmouse DNA, their glucose values appear to match with longitude/habitat.
If habitat drives glucose, as our data suggests, it may play an important role in species distribution and reduce genetic introgression across the hybrid zone. Black-crested Titmice have been observed east of the BE, but Tufted Titmice are rarely observed west of the BE [24Patten MA, Smith-Patten BD. Black-crested Titmouse (Baeolophus atricristatus). [Internet]. Cornell Lab of Ornithology 2008. Available from: http://bna.birds.cornell.edu.lib-ezproxy.tamu.edu:2048/bna/ species/717/articles/introduction, 25Ritchison G, Grubb TC Jr, Pravosudov VV. Tufted Titmouse (Baeolophus bicolor). [Internet]. Cornell Lab of Ornithology 2015. Available from: http://bna.birds.cornell.edu/bna/species/086/articles /introduction]. Tufted Titmice may have low survival west of the BE due to physiological intolerance to unpredictable food and water resources. Therefore, the physiology of glucose metabolism could act as a limiting factor to the colonization west of the BE by the Tufted Titmouse.
4.2. Influence of Age and Sex
Other venous blood analytes were influenced by age, sex, season, and sedative administration. Although saturated oxygen and base excess were higher in males, especially those administered midazolam, we suspect that the sex difference is an artificial finding based on sample size and influence of midazolam.
Both sex and midazolam administration influenced venous blood potassium concentrations. Adult females showed relatively increased venous blood potassium concentrations compared to other groups. The most common cause of hyperkalemia in human and veterinary medicine is red blood cell lysis [50Asirvatham JR, Moses V, Bjornson L. Errors in potassium measurement: A laboratory perspective for the clinician. N Am J Med Sci 2013; 5(4): 255-9.
[http://dx.doi.org/10.4103/1947-2714.110426] [PMID: 23724399] ]. However, in avian species, the effect of hemolysis on potassium concentrations has not been evaluated to our knowledge. In this study, however, no adult female received the sedative midazolam. Thus, adult females may have undergone more stress and struggle during sampling resulting in a sample with relatively increased cellular lysis prior to analysis. During stress or strenuous exercise, increased blood pressure and muscular contractions trigger the kidneys to release potassium to ensure adequate sodium-potassium pump activity [51Siegel HS. Physiological stress in birds. Bioscience 1980; 30(8): 529-34.
[http://dx.doi.org/10.2307/1307973] , 52Stranahan AM, Lee K, Mattson MP. Central mechanisms of HPA axis regulation by voluntary exercise. Neuromolecular Med 2008; 10(2): 118-27.
[http://dx.doi.org/10.1007/s12017-008-8027-0] [PMID: 18273712] ]. Furthermore, as all females were captured during nesting or breeding season, females could have experienced greater energetic demands, and handling and blood sampling may have been more problematic without sedation. The capture of both sexes outside the breeding season could confirm if the increased potassium concentrations in female Titmice are consistently present, or they result from increased energetic demands related to breeding. Recording a hemolysis score for the blood samples could have facilitated the determination of the effect of hemolysis, if any, upon potassium concentrations in our samples [53Yoo G, Kim J, Uh Y, Yoon KR, Park SD, Yoon KJ. Scoring system for detecting spurious hemolysis in anticoagulated blood specimens. Ann Lab Med 2015; 35(3): 341-7.
[http://dx.doi.org/10.3343/alm.2015.35.3.341] [PMID: 25932443] ].
As found in our study, juvenile passerine birds of many species often have a relatively decreased hematocrit compared to adults [54Potti J. Variation in the hematocrit of a passerine bird across life stages is mainly of environmental origin. J Avian Biol 2007; 38(6): 726-30.
[http://dx.doi.org/10.1111/j.2007.0908-8857.04073.x] ]. Hematocrit’s difference between ages is likely a product of body size [55Senar JC, Pascual J. Keel and tarsus length may provide a good predictor of avian body size. Ardea 1997; 85(2): 269-74.]. The relationship was strongest with males, as expected in Titmice, since males are larger than females [25Ritchison G, Grubb TC Jr, Pravosudov VV. Tufted Titmouse (Baeolophus bicolor). [Internet]. Cornell Lab of Ornithology 2015. Available from:
http://bna.birds.cornell.edu/bna/species/086/articles
/introduction, 27Curry CM, Patten MA. Current and historical extent of phenotypic variation in the Tufted and Black-crested titmouse (Paridae) hybrid zone in the southern Great Plains. Am Midl Nat 2014; 171(2): 271-300.
[http://dx.doi.org/10.1674/0003-0031-171.2.271] ]. Since hematocrit varies greatly between individuals due to health, hydration, life history, and more, the unexplained high variance is unsurprising. The observed difference of Hct, between sexes, might have been stronger with a larger sample size of adult females and less disparity of the ratio of juveniles and adults.
Similar to other studies, we also found a discrepancy between PCV and measured Hct, with PCV values consistently greater than Hct [30Heatley JJ, Cary J, Russell KE, Voelker G. Clinicopathological analysis of Passeriform venous blood reflects tranitions in elevation and habitat. Veterinary Medicine: Research and Reports 2013; 4: 21-9., 34Abbott Point of Care i-STAT System Manual: Hematocrit/HCT and calculated hemogloblin/HB 2016.]. The discrepancy of methods for determination avian hematocrit is likely explained by the relative difference in the avian red blood cell shape, volume and retained nucleus, compared to the human red blood cell [29Heatley JJ, Cary J, Kingsley L, Beaufrere H, Russell KE, Voelker G. Midazolam sedates Passeriformes for field sampling but affects multiple venous blood analytes. Vet Med (Auckl) 2015; 6: 61-9.
[http://dx.doi.org/10.2147/VMRR.S71402] [PMID: 30155435] , 56Yaw TJ, Gentry J, Ratliff C, et al. Venous blood analytes and osmolality of rehabilitated juvenile Black-bellied Whistling Ducks (Dendrocygna autumnalis). J Avian Med Surg 2019; 33(2): 123-32.
[http://dx.doi.org/10.1647/2016-194] [PMID: 31251499] , 57Pistone J, Heatley JJ, Campbell TA, Voelker G. Assessing Passeriformes health in South Texas via select venous analytes. Comp Biochem Physiol B Biochem Mol Biol 2017; 210: 64-71.
[http://dx.doi.org/10.1016/j.cbpb.2017.06.002] [PMID: 28630012] ]. Packed cell volume (as determined by centrifugation), rather than Hct (as determined by electrical resistance via the i-STAT 1), provides consistently lower results and should remain the gold standard for use in passerine species. Packed cell volume (PCV or Hct), the ratio of red blood cells to total blood volume expressed as a percent, can indicate dehydration or fitness based on an individual’s ability to transport oxygen [34Abbott Point of Care i-STAT System Manual: Hematocrit/HCT and calculated hemogloblin/HB 2016.]. Larger organisms usually have increased metabolic demands based on increased body and organ size and greater demand for oxygen transport [58Glazier DS. Effects of metabolic level on the body size scaling of metabolic rate in birds and mammals. Proc Biol Sci 2008; 275(1641): 1405-10.
[http://dx.doi.org/10.1098/rspb.2008.0118] [PMID: 18348961] , 59Minias P. The use of haemoglobin concentrations to assess physiological condition in birds: A review. Conserv Physiol 2015; 3(1)cov007
[http://dx.doi.org/10.1093/conphys/cov007] [PMID: 27293692] ]. In addition, adult birds and those with androgens, rather than estrogens, also tend toward relatively increased red blood cell mass with resulting increased PCV [60Johnstone CP, Lill A, Reina RD. Use of erythrocyte indicators of health and condition in vertebrate ecophysiology: A review and appraisal. Biol Rev Camb Philos Soc 2017; 92(1): 150-68.
[http://dx.doi.org/10.1111/brv.12219] [PMID: 28075072] , 61Scanes CG. Allometric and phylogenic comparisons of hematological parameters between and within birds and mammals. Int J Veterin Health Sci Res 2016; 4(5): 123-9.]. However, Hct should be further evaluated in these species with a larger adult sample size and with individuals from across the full distribution of the species to ensure other factors, such as latitude or elevation, are not influencing Hct or PCV.
4.3. Effect of Midazolam and Handling Upon Venous Blood Analytes
Administration of midazolam affected many blood gas, and electrolyte values often used to determine health in wild birds. Interpretation of avian health in wild birds is inherently complicated as capture, handling, and blood collection are stressful events for free-living birds [62Le Maho Y, Karmann H, Briot D, et al. Stress in birds due to routine handling and a technique to avoid it. Am J Physiol 1992; 263(4 Pt 2): R775-81.
[PMID: 1415787] -64Newman SH, Carter HR, Whitworth DL, Zinkl JG. Health assessments and stress response of Xantus’s murrelets to capture, handling, and radio-marking. Mar Ornithol 2005; 33: 147-54.]. Physicians and dentists have used midazolam for decades to reduce procedural stress in children [65Kupietzky A, Houpt MI. Midazolam: A review of its use for conscious sedation of children. Pediatr Dent 1993; 15(4): 237-41.
[PMID: 8247896] , 66Lee-Kim SJ, Fadavi S, Punwani I, Koerber A. Nasal versus oral midazolam sedation for pediatric dental patients. J Dent Child (Chic) 2004; 71(2): 126-30.
[PMID: 15587094] ]. Midazolam is a benzodiazepine that boosts inhibitory neurotransmitters, decreases neuron excitability and results in sedation, anxiolysis, and/or hypnosis depending upon the dose administered [65Kupietzky A, Houpt MI. Midazolam: A review of its use for conscious sedation of children. Pediatr Dent 1993; 15(4): 237-41.
[PMID: 8247896] ]. However, hypoxia, reduced respiration, reduced memory, and stimulation of a stress endocrine response may also occur [29Heatley JJ, Cary J, Kingsley L, Beaufrere H, Russell KE, Voelker G. Midazolam sedates Passeriformes for field sampling but affects multiple venous blood analytes. Vet Med (Auckl) 2015; 6: 61-9.
[http://dx.doi.org/10.2147/VMRR.S71402] [PMID: 30155435] , 65Kupietzky A, Houpt MI. Midazolam: A review of its use for conscious sedation of children. Pediatr Dent 1993; 15(4): 237-41.
[PMID: 8247896] ]. In multiple avian species, the intranasal administration of midazolam has been shown to reduce the stress of handling and venipuncture [67Vesal N, Eskandari MH. Sedative effects of midazolam and xylazine with or without ketamine and detomidine alone following intranasal administration in Ring-necked Parakeets. J Am Vet Med Assoc 2006; 228(3): 383-8.
[http://dx.doi.org/10.2460/javma.228.3.383] [PMID: 16448361] ].
In our study, midazolam administration influenced all analytes we measured in Titmice except pH, Hct, and Hb. For both species, irrespective of geographic location, Titmice given midazolam had, on average, higher pCO2, HCO3, tCO2, base excess, glucose, and sodium values. Midazolam lowered the venous partial pressure of oxygen (pO2), and oxygen saturation (sO2), as well as concentrations of lactate, potassium, chloride, and BUN. Except for glucose, changes in all analytes paralleled those seen following the administration of midazolam to humans [68Tucker MR, Ochs MW, White RP Jr. Arterial blood gas levels after midazolam or diazepam administered with or without fentanyl as an intravenous sedative for outpatient surgical procedures. J Oral Maxillofac Surg 1986; 44(9): 688-92.
[http://dx.doi.org/10.1016/0278-2391(86)90036-4] [PMID: 2943882] ].
While the location of capture was a stronger predictor of blood glucose concentrations than sedative administration, glucose concentrations were unexpectedly higher in birds receiving midazolam. Gluconeogenesis occurs near the end of the stress response pathway to meet the increased energetic demands of the stressor [51Siegel HS. Physiological stress in birds. Bioscience 1980; 30(8): 529-34.
[http://dx.doi.org/10.2307/1307973] , 69Blas J. Stress in Birds.Sturkie’s Avian Physiology 6th ed. 2015; 769-810.
[http://dx.doi.org/10.1016/B978-0-12-407160-5.00033-6] ]. Production of glucose is a vital component of the negative feedback mechanism regulating the sympathetic (aka “fight or flight”) nervous system. Even after a stress stimulus is removed, glucose concentrations may continue to rise based on the time lag between neuroendocrine communication and the hepatic metabolic response [51Siegel HS. Physiological stress in birds. Bioscience 1980; 30(8): 529-34.
[http://dx.doi.org/10.2307/1307973] , 70Braun EJ, Sweazea KL. Glucose regulation in birds. Comp Biochem Physiol B Biochem Mol Biol 2008; 151(1): 1-9.
[http://dx.doi.org/10.1016/j.cbpb.2008.05.007] [PMID: 18571448] ]. Mist-net capture, handling, and blood sampling each induce stress on wild birds, so increased glucose concentrations were expected in birds [64Newman SH, Carter HR, Whitworth DL, Zinkl JG. Health assessments and stress response of Xantus’s murrelets to capture, handling, and radio-marking. Mar Ornithol 2005; 33: 147-54., 71Harms CA, Harms RV. Venous blood gas and lactate values of mourning doves (Zenaida macroura), boat-tailed grackles (Quiscalus major), and house sparrows (Passer domesticus) after capture by mist net, banding, and venipuncture. J Zoo Wildl Med 2012; 43(1): 77-84.
[http://dx.doi.org/10.1638/2011-0114.1] [PMID: 22448512] , 72Harms CA, Jinks MR, Harms RV. Blood gas, lactate, and hematology effects of venipuncture timing and location after mist-net capture of Mourning Doves (Zenaida macroura), Boat-tailed Grackles (Quiscalus major), and House Sparrows (Passer domesticus). J Wildl Dis 2016; 52(2)(Suppl.): S54-64.
[http://dx.doi.org/10.7589/52.2S.S54] [PMID: 26845300] ]. The apparent ineffectiveness of midazolam to reduce glucose concentrations is likely a product of the lag time between capture until midazolam administration. Although we obtained blood 0-5 min following sedation, a stressful event (mist-net capture) has already occurred prior to anxiolytic administration. Further stress, from handling, during sedative administration, could also cause blood glucose concentrations to continue increasing during blood collection despite sedative administration. To better assess the efficacy of midazolam for stress reduction in captured birds, future studies could measure blood glucose concentrations and or corticosterone at various time intervals (e.g., 15-60 min) following capture handling and midazolam administration. Although midazolam changed multiple venous blood analytes similarly to humans experiencing anxiolysis, this sedative’s impact on blood gases and electrolytes in passerine birds needs further investigation.
We administrated midazolam to titmice in 2012 to reduce stress and facilitate blood collection while assessing health. Providing a rest period after capture and before blood collection may also reduce stress, as indicated by blood gases and other variables [71Harms CA, Harms RV. Venous blood gas and lactate values of mourning doves (Zenaida macroura), boat-tailed grackles (Quiscalus major), and house sparrows (Passer domesticus) after capture by mist net, banding, and venipuncture. J Zoo Wildl Med 2012; 43(1): 77-84.
[http://dx.doi.org/10.1638/2011-0114.1] [PMID: 22448512] , 72Harms CA, Jinks MR, Harms RV. Blood gas, lactate, and hematology effects of venipuncture timing and location after mist-net capture of Mourning Doves (Zenaida macroura), Boat-tailed Grackles (Quiscalus major), and House Sparrows (Passer domesticus). J Wildl Dis 2016; 52(2)(Suppl.): S54-64.
[http://dx.doi.org/10.7589/52.2S.S54] [PMID: 26845300] ]. Blood samples from Mourning Doves (Zenaida macroura), Boat-tailed Grackles (Quiscalus major), and House Sparrows (Passer domesticus), immediately after capture or after a 45-60 min delay, lacked differences in pH for any species, while blood gas values (pCO2, pO2, and HCO3-) and PCV varied based on species. However, lactate significantly decreased based on a rest period in all species. We found similar results in Titmice when comparing birds receiving a sedative to those without the sedative. However, this comparison should be reviewed with caution, as the authors studied two Passeriformes species and a Columbiformes species that were larger than the Titmice of this study [72Harms CA, Jinks MR, Harms RV. Blood gas, lactate, and hematology effects of venipuncture timing and location after mist-net capture of Mourning Doves (Zenaida macroura), Boat-tailed Grackles (Quiscalus major), and House Sparrows (Passer domesticus). J Wildl Dis 2016; 52(2)(Suppl.): S54-64.
[http://dx.doi.org/10.7589/52.2S.S54] [PMID: 26845300] ].
CONCLUSION
Titmice living west of the Balcones Escarpment have higher venous blood glucose concentrations than individuals east of the BE. Glucose values were further elevated following rainfall west of the BE. We suspect that naturally increased glucose values are due to chronic stress from unpredictable food sources in a semi-arid environment. Naturally higher glucose concentrations, in western birds, are supported by a further increase in their glucose concentrations following rainfall, an event representing a change in their environment. In arid and semi-arid environments, such as the BE, sporadic rainfall stimulates primary productivity, which can induce hyperphagia and/or increase metabolic activity, both of which are known causes of increased glucose. We recommend controlled experiments to investigate the behavior, physiology, and fitness of both species in opposing natural habitats. Such studies could provide evidence of hybrid zone reinforcement and possible hybrid zone movement or range expansion. Until data collection and knowledge advance to provide complete physiological reference data for appropriate indicator species of study, we, as wildlife biologists, must strive to continue increasing our understanding of avian physiology through comparative studies between species, updating handling protocols to include use of sedatives, understand and include environmental conditions in our study design, as well as investigating the impact of physiological differences between populations and species, especially as it relates to habitat effects.
LIST OF ABBREVIATIONS
| BE | = Balcones Escarpment |
| PCV | = Packed Cell Volume |
| Hct | = Hematocrit |
| Hb | = Hemoglobin |
| ANOVA | = Analysis of Variance |
AUTHORS' CONTRIBUTIONS
J.C.V and G.V. conceived the idea, design, and experiment; J.C.V and J.J.H. collected data, conducted research; J.C.V. wrote the paper; J.C.V. and J.J.H. designed the methods; J.C.V. analyzed the data; J.J.H. and G.V. contributed materials, resources, funding, and edited the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This research was conducted under required Texas Parks and Wildlife (permit# SPR-0209-016) and United States Fish and Wildlife Permits (permit# 188 MB205752) and with approval of the Texas A&M University Institutional Animal Care and Use Committee (AUP# 2012-6).
HUMAN AND ANIMAL RIGHTS
No humans were used for studies that are the basis of this research. All animal experiments were conducted in accordance with the Guidelines for euthanasia and ethical authorization of the study, all got approved from the Board of the Texas A&M University Institutional Animal Care and Use Committee (AUP# 2012-6).
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available at [ https://vetmed.tamu.edu/schubot/research/publications/ ], reference number [publication # SC 161].
FUNDING
Non-competitive supporting funds provided by the Schubot Exotic Bird Health Center and the College of Veterinary Medicine and Biomedical Sciences.
CONFLICT OF INTEREST
J.C.V.: none; G.V.: none; J.J.H.: none
ACKNOWLEDGEMENTS
This research was part of a Texas Ecolab Grant through Braun & Gresham, PLLC. We thank the following landowners for funding the Ecolab program, and for access to their properties: Gail Allen, Megan Biesele, June Dezendorf, John Evancho, Steven Fehrenkamp, Stuart Kensinger, Doug Kelsay, John Panak, and Benjamin Rivera, Allen Simons, Trudy Verser. We also would like to thank the Willis and Theode families for their support and hospitality. This is publication number 1617 of the Biodiversity Research and Teaching Collections, at Texas A&M University. We thank Schubot Exotic Bird Health Center, College of Veterinary Medicine and Biomedical Sciences for their financial and technical support.
REFERENCES
| [1] | Barton NH, Hewitt GM. Analysis of hybrid zones. Annu Rev Ecol Syst 1985; 16: 113-48. [http://dx.doi.org/10.1146/annurev.es.16.110185.000553] |
| [2] | Abbott R, Albach D, Ansell S, et al. Hybridization and speciation. J Evol Biol 2013; 26(2): 229-46. [http://dx.doi.org/10.1111/j.1420-9101.2012.02599.x] [PMID: 23323997] |
| [3] | Barton NH. The role of hybridization in evolution. Mol Ecol 2001; 10(3): 551-68. [http://dx.doi.org/10.1046/j.1365-294x.2001.01216.x] [PMID: 11298968] |
| [4] | Swenson NG, Howard DJ. Clustering of contact zones, hybrid zones, and phylogeographic breaks in North America. Am Nat 2005; 166(5): 581-91. [http://dx.doi.org/10.1086/491688] [PMID: 16224723] |
| [5] | Miller MJ, Lipshutz SE, Smith NG, Bermingham E. Genetic and phenotypic characterization of a hybrid zone between polyandrous Northern and Wattled Jacanas in Western Panama. BMC Evol Biol 2014; 14(1): 227. [http://dx.doi.org/10.1186/s12862-014-0227-7] [PMID: 25394718] |
| [6] | Curry CM. An integrated framework for hybrid zone models. Evol Biol 2015; 42(3): 359-65. [http://dx.doi.org/10.1007/s11692-015-9332-9] |
| [7] | Semenov GA, Scordato ESC, Khaydarov DR, Smith CCR, Kane NC, Safran RJ. Effects of assortative mate choice on the genomic and morphological structure of a hybrid zone between two bird subspecies. Mol Ecol 2017; 26(22): 6430-44. [http://dx.doi.org/10.1111/mec.14376] [PMID: 28987006] |
| [8] | Harrison RG, Larson EL. Hybridization, introgression, and the nature of species boundaries. J Hered 2014; 105(S1)(Suppl. 1): 795-809. [http://dx.doi.org/10.1093/jhered/esu033] [PMID: 25149255] |
| [9] | Eme D, Malard F, Colson-Proch C, et al. Integrating phylogeography, physiology and habitat modelling to explore species range determinants. J Biogeogr 2014; 41(4): 687-99. [http://dx.doi.org/10.1111/jbi.12237] |
| [10] | Kotlik P, Markova S, Vojtek L, Stratil A. Adaptive phylogeography: functional divergence between haemoglobins derived from different glacial refugia in the bank vole. Proceedings of the Royal Society B: Biological Sciences 2014; 281(1786): 20140021. |
| [11] | Endler JA. Geographic Variation, Speciation, and Clines 1977. |
| [12] | Bozinovic F, Naya DE. Linking Physiology, Climate, and Species Distributional Ranges.Integrative Organismal Biology 1st ed. 2015; 14. |
| [13] | Culumber ZW, Shepard DB, Coleman SW, Rosenthal GG, Tobler M. Physiological adaptation along environmental gradients and replicated hybrid zone structure in swordtails (Teleostei: Xiphophorus). J Evol Biol 2012; 25(9): 1800-14. [http://dx.doi.org/10.1111/j.1420-9101.2012.02562.x] [PMID: 22827312] |
| [14] | DuBay SG, Witt CC. Differential high-altitude adaptation and restricted gene flow across a mid-elevation hybrid zone in Andean tit-tyrant flycatchers. Mol Ecol 2014; 23(14): 3551-65. [http://dx.doi.org/10.1111/mec.12836] [PMID: 24943893] |
| [15] | Cadenasso ML, Pickett STA, Weathers KC, Jones CG. A framework for a theory of ecological boundaries. Bioscience 2003; 53(8): 750-8. [http://dx.doi.org/10.1641/0006-3568(2003)053[0750:AFFATO]2.0.CO;2] |
| [16] | Abbott P, Woodruff CM Jr. The balcones escarpment: Geology, hydrology, ecology, and social development in Central Texas 1986. |
| [17] | Griffith G, Bryce S, Omernick J, Rogers A. Cartographers. Ecoregions of Texas 2007. |
| [18] | Webb WL. Biogeographic regions of Texas and Oklahoma. Ecology 1950; 31(3): 426-33. [http://dx.doi.org/10.2307/1931496] |
| [19] | Goetze JR. Distribution, natural history, and biogeographic relationships of mammals on the Edwards Plateau of Texas 1995. |
| [20] | Hibbitts TJ, Ryberg WA, Harvey JA, et al. Phylogenetic structure of Holbrookia lacerata (Cope 1880) (Squamata: Phrynosomatidae): one species or two? Zootaxa 2019; 4619(1): 6. [http://dx.doi.org/10.11646/zootaxa.4619.1.6] [PMID: 31716318] |
| [21] | Owen JG, Dixon JR. An ecogeographic analysis of the herpetofauna of Texas. Southwest Nat 1989; 34(2): 165-80. [http://dx.doi.org/10.2307/3671726] |
| [22] | Smith HM, Buechner HK. The influence of the Balcones Escarpment on the distribution of amphibians and reptiles in Texas. Bullentin of the Chicago Academy of Sciences 1947; 8(1): 1-16. |
| [23] | Dixon KL. Contact zones of avian congeners on the southern Great Plains. Condor 1989; 91(1): 15-22. [http://dx.doi.org/10.2307/1368143] |
| [24] | Patten MA, Smith-Patten BD. Black-crested Titmouse (Baeolophus atricristatus). [Internet]. Cornell Lab of Ornithology 2008. Available from: http://bna.birds.cornell.edu.lib-ezproxy.tamu.edu:2048/bna/ species/717/articles/introduction |
| [25] | Ritchison G, Grubb TC Jr, Pravosudov VV. Tufted Titmouse (Baeolophus bicolor). [Internet]. Cornell Lab of Ornithology 2015. Available from: http://bna.birds.cornell.edu/bna/species/086/articles /introduction |
| [26] | Dixon KL. An ecological analysis of the inter-breeding of crested titmice in Texas. Univ Calif Publ Zool 1955; 54(3): 125-206. |
| [27] | Curry CM, Patten MA. Current and historical extent of phenotypic variation in the Tufted and Black-crested titmouse (Paridae) hybrid zone in the southern Great Plains. Am Midl Nat 2014; 171(2): 271-300. [http://dx.doi.org/10.1674/0003-0031-171.2.271] |
| [28] | Curry CM, Patten MA. Shadow of a doubt: premating and postmating isolating barriers in a temporally complex songbird (Passeriformes: Paridae) hybrid zone. Behav Ecol Sociobiol 2016; 70(8): 1171-86. [http://dx.doi.org/10.1007/s00265-016-2126-y] |
| [29] | Heatley JJ, Cary J, Kingsley L, Beaufrere H, Russell KE, Voelker G. Midazolam sedates Passeriformes for field sampling but affects multiple venous blood analytes. Vet Med (Auckl) 2015; 6: 61-9. [http://dx.doi.org/10.2147/VMRR.S71402] [PMID: 30155435] |
| [30] | Heatley JJ, Cary J, Russell KE, Voelker G. Clinicopathological analysis of Passeriform venous blood reflects tranitions in elevation and habitat. Veterinary Medicine: Research and Reports 2013; 4: 21-9. |
| [31] | Mans C, Guzman DSM, Lahner LL, Paul-Murphy J, Sladky KK. Sedation and physiologic response to manual restraint after intranasal administration of midazolam in Hispaniolan Amazon parrots (Amazona ventralis). J Avian Med Surg 2012; 26(3): 130-9. [http://dx.doi.org/10.1647/2011-037R.1] [PMID: 23156974] |
| [32] | Bigham AS, Zamani Moghaddam AK. Finch (Taeneopygia guttata) sedation with intranasal administration of diazepam, midazolam or xylazine. J Vet Pharmacol Ther 2013; 36(1): 102-4. [http://dx.doi.org/10.1111/j.1365-2885.2009.01102.x] [PMID: 23317426] |
| [33] | Schaffer DPH, de Araújo NLLC, Raposo ACS, Filho EFM, Vieira JVR, Oriá AP. Sedative effects of intranasal midazolam administration in wild caught Blue-fronted Amazon (Amazona aestiva) and Orange-winged Amazon (Amazona amazonica) Parrots. J Avian Med Surg 2017; 31(3): 213-8. [http://dx.doi.org/10.1647/2016-201] [PMID: 28891701] |
| [34] | Abbott Point of Care i-STAT System Manual: Hematocrit/HCT and calculated hemogloblin/HB 2016. |
| [35] | Abbott Point of Care i-STAT System Manual: PCO2 and calculated values for HCO3, base excess and anion gap 2017. |
| [36] | Lill A. Sources of variation in blood glucose concentrations of free-living birds. Avian Biol Res 2011; 4(2): 78-86. [http://dx.doi.org/10.3184/175815511X13073729328092] |
| [37] | Tomasek O, Bobek L, Kralova T, Adamkova M, Albrecht T. Fuel for the pace of life: Baseline blood glucose concentration co-evolves with life-history traits in songbirds. Funct Ecol 2019; 33(2): 239-49. [http://dx.doi.org/10.1111/1365-2435.13238] |
| [38] | Jimeno B, Hau M, Verhulst S. Corticosterone levels reflect variation in metabolic rate, independent of ‘stress’. Sci Rep 2018; 8(1): 13020. [http://dx.doi.org/10.1038/s41598-018-31258-z] [PMID: 30158537] |
| [39] | Kaliński A, Bańbura M, Glądalski M, et al. Long-term variation in blood glucose concentration in nestling Great Tits (Parus major). Avian Biol Res 2015; 8(3): 129-37. [http://dx.doi.org/10.3184/175815515X14294426911072] |
| [40] | Hau M, Casagrande S, Ouyang JQ, Baugh AT. Chapter two - glucocorticoid-mediated phenotypes in vertebrates: Multilevel variation and evolution advances in the study of behavior 48 2016; 41-115. |
| [41] | Nielsen-Gammon JW. Changing climate of texas. Impact of global warming on texas 2nd ed. 2011; 39-68. |
| [42] | Heilman JL, McInnes KJ, Kjelgaard JF, Keith Owens M, Schwinning S. Energy balance and water use in a subtropical karst woodland on the Edwards Plateau, Texas. J Hydrol (Amst) 2009; 373(3): 426-35. [http://dx.doi.org/10.1016/j.jhydrol.2009.05.007] |
| [43] | Yang LH, Bastow JL, Spence KO, Wright AN. What can we learn from resource pulses? Ecology 2008; 89(3): 621-34. [http://dx.doi.org/10.1890/07-0175.1] [PMID: 18459327] |
| [44] | Cherel Y, Robin J-P, Maho YL. Physiology and biochemistry of long-term fasting in birds. Can J Zool 1988; 66(1): 159-66. [http://dx.doi.org/10.1139/z88-022] |
| [45] | Kitaysky AS, Piatt JF, Wingfield JC. Stress hormones link food availability and population processes in seabirds. Mar Ecol Prog Ser 2007; 352: 245-58. [http://dx.doi.org/10.3354/meps07074] |
| [46] | Fokidis HB, des Roziers MB, Sparr R, Rogowski C, Sweazea K, Deviche P. Unpredictable food availability induces metabolic and hormonal changes independent of food intake in a sedentary songbird. J Exp Biol 2012; 215(Pt 16): 2920-30. [http://dx.doi.org/10.1242/jeb.071043] [PMID: 22837467] |
| [47] | Williams J, Tieleman BI. Physiological ecology and behavior of desert birds.Current Ornithology Current Ornithology 16 2001; 299-353. [http://dx.doi.org/10.1007/978-1-4615-1211-0_6] |
| [48] | Ostfeld RS, Keesing F. Pulsed resources and community dynamics of consumers in terrestrial ecosystems. Trends Ecol Evol (Amst) 2000; 15(6): 232-7. [http://dx.doi.org/10.1016/S0169-5347(00)01862-0] [PMID: 10802548] |
| [49] | Wenninger EJ, Inouye RS. Insect community response to plant diversity and productivity in a sagebrush–steppe ecosystem. J Arid Environ 2008; 72(1): 24-33. [http://dx.doi.org/10.1016/j.jaridenv.2007.04.005] |
| [50] | Asirvatham JR, Moses V, Bjornson L. Errors in potassium measurement: A laboratory perspective for the clinician. N Am J Med Sci 2013; 5(4): 255-9. [http://dx.doi.org/10.4103/1947-2714.110426] [PMID: 23724399] |
| [51] | Siegel HS. Physiological stress in birds. Bioscience 1980; 30(8): 529-34. [http://dx.doi.org/10.2307/1307973] |
| [52] | Stranahan AM, Lee K, Mattson MP. Central mechanisms of HPA axis regulation by voluntary exercise. Neuromolecular Med 2008; 10(2): 118-27. [http://dx.doi.org/10.1007/s12017-008-8027-0] [PMID: 18273712] |
| [53] | Yoo G, Kim J, Uh Y, Yoon KR, Park SD, Yoon KJ. Scoring system for detecting spurious hemolysis in anticoagulated blood specimens. Ann Lab Med 2015; 35(3): 341-7. [http://dx.doi.org/10.3343/alm.2015.35.3.341] [PMID: 25932443] |
| [54] | Potti J. Variation in the hematocrit of a passerine bird across life stages is mainly of environmental origin. J Avian Biol 2007; 38(6): 726-30. [http://dx.doi.org/10.1111/j.2007.0908-8857.04073.x] |
| [55] | Senar JC, Pascual J. Keel and tarsus length may provide a good predictor of avian body size. Ardea 1997; 85(2): 269-74. |
| [56] | Yaw TJ, Gentry J, Ratliff C, et al. Venous blood analytes and osmolality of rehabilitated juvenile Black-bellied Whistling Ducks (Dendrocygna autumnalis). J Avian Med Surg 2019; 33(2): 123-32. [http://dx.doi.org/10.1647/2016-194] [PMID: 31251499] |
| [57] | Pistone J, Heatley JJ, Campbell TA, Voelker G. Assessing Passeriformes health in South Texas via select venous analytes. Comp Biochem Physiol B Biochem Mol Biol 2017; 210: 64-71. [http://dx.doi.org/10.1016/j.cbpb.2017.06.002] [PMID: 28630012] |
| [58] | Glazier DS. Effects of metabolic level on the body size scaling of metabolic rate in birds and mammals. Proc Biol Sci 2008; 275(1641): 1405-10. [http://dx.doi.org/10.1098/rspb.2008.0118] [PMID: 18348961] |
| [59] | Minias P. The use of haemoglobin concentrations to assess physiological condition in birds: A review. Conserv Physiol 2015; 3(1)cov007 [http://dx.doi.org/10.1093/conphys/cov007] [PMID: 27293692] |
| [60] | Johnstone CP, Lill A, Reina RD. Use of erythrocyte indicators of health and condition in vertebrate ecophysiology: A review and appraisal. Biol Rev Camb Philos Soc 2017; 92(1): 150-68. [http://dx.doi.org/10.1111/brv.12219] [PMID: 28075072] |
| [61] | Scanes CG. Allometric and phylogenic comparisons of hematological parameters between and within birds and mammals. Int J Veterin Health Sci Res 2016; 4(5): 123-9. |
| [62] | Le Maho Y, Karmann H, Briot D, et al. Stress in birds due to routine handling and a technique to avoid it. Am J Physiol 1992; 263(4 Pt 2): R775-81. [PMID: 1415787] |
| [63] | Carere C, van Oers K. Shy and bold great tits (Parus major): Body temperature and breath rate in response to handling stress. Physiol Behav 2004; 82(5): 905-12. [http://dx.doi.org/10.1016/S0031-9384(04)00312-9] [PMID: 15451657] |
| [64] | Newman SH, Carter HR, Whitworth DL, Zinkl JG. Health assessments and stress response of Xantus’s murrelets to capture, handling, and radio-marking. Mar Ornithol 2005; 33: 147-54. |
| [65] | Kupietzky A, Houpt MI. Midazolam: A review of its use for conscious sedation of children. Pediatr Dent 1993; 15(4): 237-41. [PMID: 8247896] |
| [66] | Lee-Kim SJ, Fadavi S, Punwani I, Koerber A. Nasal versus oral midazolam sedation for pediatric dental patients. J Dent Child (Chic) 2004; 71(2): 126-30. [PMID: 15587094] |
| [67] | Vesal N, Eskandari MH. Sedative effects of midazolam and xylazine with or without ketamine and detomidine alone following intranasal administration in Ring-necked Parakeets. J Am Vet Med Assoc 2006; 228(3): 383-8. [http://dx.doi.org/10.2460/javma.228.3.383] [PMID: 16448361] |
| [68] | Tucker MR, Ochs MW, White RP Jr. Arterial blood gas levels after midazolam or diazepam administered with or without fentanyl as an intravenous sedative for outpatient surgical procedures. J Oral Maxillofac Surg 1986; 44(9): 688-92. [http://dx.doi.org/10.1016/0278-2391(86)90036-4] [PMID: 2943882] |
| [69] | Blas J. Stress in Birds.Sturkie’s Avian Physiology 6th ed. 2015; 769-810. [http://dx.doi.org/10.1016/B978-0-12-407160-5.00033-6] |
| [70] | Braun EJ, Sweazea KL. Glucose regulation in birds. Comp Biochem Physiol B Biochem Mol Biol 2008; 151(1): 1-9. [http://dx.doi.org/10.1016/j.cbpb.2008.05.007] [PMID: 18571448] |
| [71] | Harms CA, Harms RV. Venous blood gas and lactate values of mourning doves (Zenaida macroura), boat-tailed grackles (Quiscalus major), and house sparrows (Passer domesticus) after capture by mist net, banding, and venipuncture. J Zoo Wildl Med 2012; 43(1): 77-84. [http://dx.doi.org/10.1638/2011-0114.1] [PMID: 22448512] |
| [72] | Harms CA, Jinks MR, Harms RV. Blood gas, lactate, and hematology effects of venipuncture timing and location after mist-net capture of Mourning Doves (Zenaida macroura), Boat-tailed Grackles (Quiscalus major), and House Sparrows (Passer domesticus). J Wildl Dis 2016; 52(2)(Suppl.): S54-64. [http://dx.doi.org/10.7589/52.2S.S54] [PMID: 26845300] |