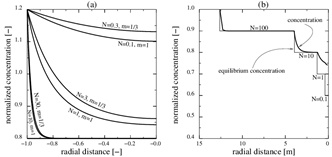
Fig. (2) (a) The flow-limited regime is N>>1 and the precipitation limited-regime is N<<1 Kinetics with m=1/3 is a good approximation for kinetics with m=1, when the Damköhler-number is increased by a factor of 3. (b) The transition from flow-limited to reaction-limited concentration when the equilibrium concentration decreases in a step-wise manner towards the well. The concentration is close to the equilibrium concentration when the characteristic length gives N>>1. The concentration does not follow the equilibrium concentration close to the well where N<<1.