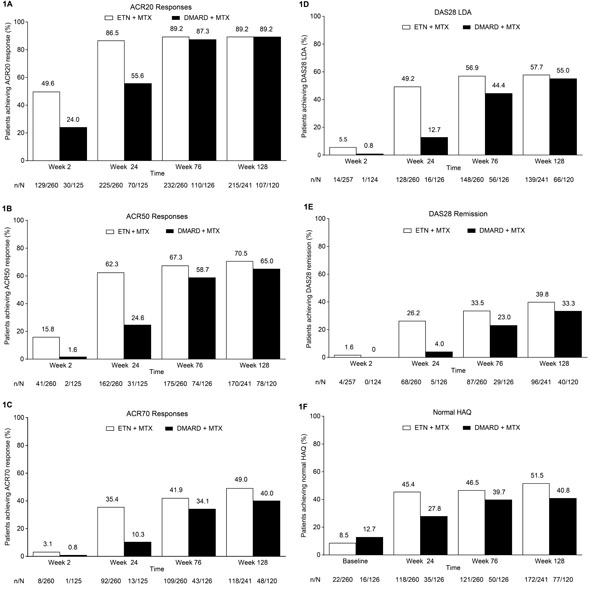
Fig. (1)
Proportion of patients achieving (A) ACR20 response, (B) ACR50
response, (C) ACR70 response, (D) DAS28 LDA, (E) DAS28
remission, and (F) normal HAQ (≤0.5). Treatment modification was
permitted at the start of the extension (i.e., after week 24); during the
extension, 259/260 patients in the ETN + MTX group continued to receive
etanercept and 105/126 patients in the DMARD + MTX group received etanercept.
ACR, American College of Rheumatology; DAS28, Disease Activity Score in 28
joints; DMARD, disease-modifying antirheumatic drug; ETN, etanercept; HAQ,
Health Assessment Questionnaire; LDA, low disease activity; LOCF, last
observation carried forward; MTX, methotrexate. Analyses included patients who
received at least one dose of study drug in the extension phase; LOCF.