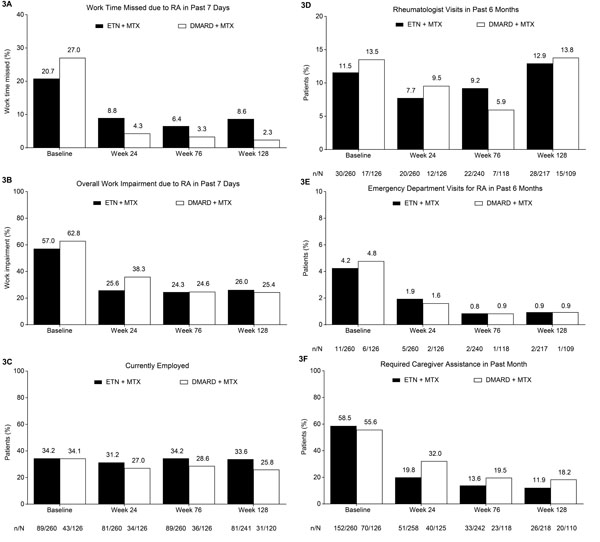
Fig. (3)
The percentage of (A) work time missed and (B) overall work
impairment due to RA in the past 7 days based on patient responses on the
WPAI:RA questionnaire. Proportions of patients who are (C) currently
employed; who have required (D) a rheumatologist visit or (E) an
emergency department visit in the past 6 months; and who have required (F)
caregiver assistance in the past month based on responses on the CBRU
questionnaire. Treatment modification was permitted at the start of the
extension (i.e., after week 24); during the extension, 259/260 patients in the
ETN + MTX group continued to receive etanercept and 105/126 patients in the
DMARD + MTX group received etanercept. Analyses included patients who received
at least one dose of study drug in the extension phase. WPAI:RA and CBRU
employment findings analyzed using LOCF; all other CBRU findings based on
observed cases. CBRU, caregiver burden and resource utilization questionnaire;
DMARD, disease-modifying anti-rheumatic drug; ETN, etanercept; LOCF, last
observation carried forward; MTX, methotrexate; RA, rheumatoid arthritis;
WPAI:RA, work productivity and activity impairment: rheumatoid arthritis
questionnaire.