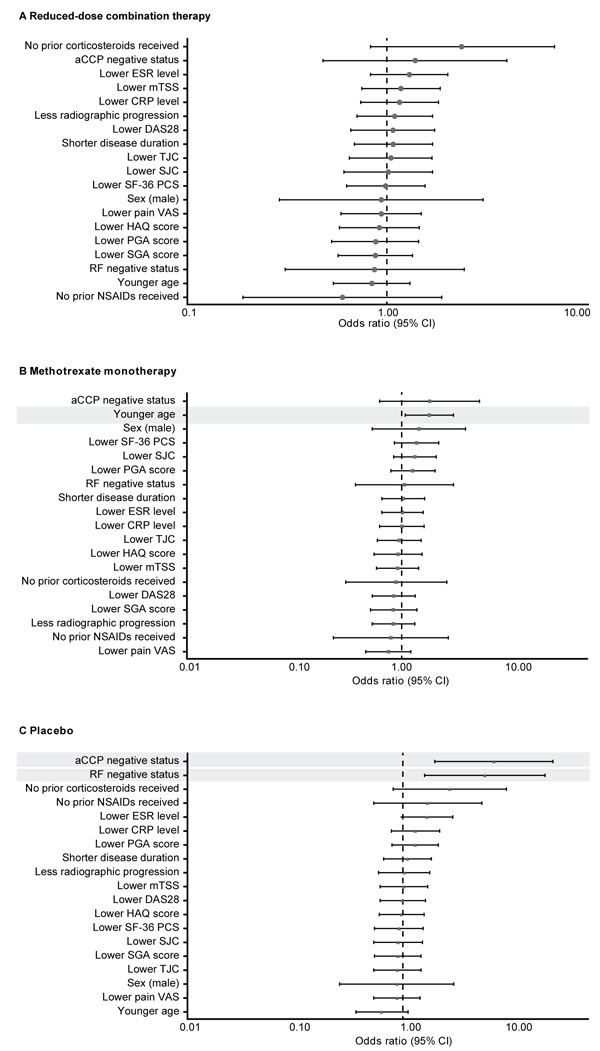
Fig. (2)
Demographic and disease characteristics at baseline as predictors of sustained remission in patients who received (A) reduced-dose combination therapy, (B) methotrexate monotherapy, and (C) placebo in the double-blind phase (double-blind mITT population). aCCP: anticyclic citrullinated peptide antibody; CI: confidence interval; CRP: C-Reactive Protein; DAS28: Disease Activity Score for 28-joint counts; ESR: Erythrocyte Sedimentation Rate; HAQ: Health Assessment Questionnaire; mITT: modified intent-to-treat; mTSS: modified Total Sharp Score; NSAID: Nonsteroidal Anti-Inflammatory Drug; PCS: Physician Component Summary; PGA: Physician Global Assessment; RF: Rheumatoid Factor; SF-36: Medical Outcomes Short Form-36 Health Survey; SGA: Subject Global Assessment ; SJC: Swollen Joint Count; TJC: Tender Joint Count; VAS: Visual Analog Scale.