- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Translational Medicine Journal
(Discontinued)
ISSN: 1876-3995 ― Volume 3, 2014
Postprandial Lipid Metabolism: The Missing Link Between Life-Style Habits and the Increasing Incidence of Metabolic Diseases in Western Countries?
Elena Bravo1, * , Mariarosaria Napolitano 1, Kathleen M. Botham2
Abstract
Postprandial lipemia is the transient increase in blood lipids which occurs after a meal containing fat and is caused by raised levels of triglyceride-rich lipoproteins (TRLs) in the blood. Delayed clearance of TRLs leads to postprandial hyperlipidemia, and there is now a great deal of evidence to support the idea that this condition is an important risk factor for cardiovascular disease (CVD). Western lifestyle habits including: diets low in fresh fruit and vegetables and high in fat and processed food, alcohol consumption, smoking, and lack of exercise tend to promote postprandial hyperlipidemia, and it is a characteristic feature of increasingly common metabolic diseases such as obesity, insulin resistance and type 2 diabetes which are also linked to modern lifestyle behaviour and which carry an increased risk of CVD development. Modification of lifestyle factors such as changing to a healthier diet, weight loss, reducing alcohol consumption and increasing exercise can cause significant reductions in postprandial hyperlipidemia and thus help to reduce this risk. Despite the growing recognition that the extent of postprandial lipemia is a good predictor of CVD, no standardized methodology for its measurement is currently available. Determination of blood TG levels after consumption of a standard test meal is likely to be the most convenient approach for a routine clinical test, and we propose a standard test meal which is easily adaptable for the variations in dietary habits in different countries. Greater use of postprandial lipid determination will aid in the translation of our extensive knowledge on the role of nutrition in health into national and international policy.
Article Information
Identifiers and Pagination:
Year: 2010Volume: 2
First Page: 1
Last Page: 13
Publisher Id: TOTRANSMJ-2-1
DOI: 10.2174/1876399501002010001
Article History:
Received Date: 30/9/2009Revision Received Date: 17/12/2009
Acceptance Date: 26/1/2010
Electronic publication date: 30 /3/2010
Collection year: 2010
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Department of Cellular Biology and Neuroscience, Istituto Superiore di Sanità, Viale Regina Elena 299 00161 Rome, Italy; Tel: +3906 4990 3061 // 2730 / 2867; Fax: +3906 4938 7143; E-mail: bravo@iss.it
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 30-9-2009 |
Original Manuscript | Postprandial Lipid Metabolism: The Missing Link Between Life-Style Habits and the Increasing Incidence of Metabolic Diseases in Western Countries? | |
INTRODUCTION
Cardiovascular disease (CVD) is the main cause of death in Western countries, accounting for over 42% of the total each year in the European Union (EU) [1] and for 34.2% in the United States (data for 2006) [2]. The main forms of CVD are coronary heart disease (CHD) and stroke. CHD is the single most common cause of death in Western countries. Data for the EU, shown in Table 1, indicate that approximately one in six (16%) men and more than one in seven (15%) women die from this disease, while stroke, the second most common cause of death, kills about 9% of men and 12% of women. In the United States (data for 2005), CHD caused about 1 in every 5 deaths and stroke about 1 in every 7.5 deaths [2].
The burden of CVD (illness and death) can be measured using the disability adjusted life year (DALY), which is an aggregate of the years of life lost due to premature death and years of healthy life lost because of disability. In the EU, 19% of the total DALYs are lost because of CVD (Table 1). Moreover, in addition to the human costs there are major economic costs, estimated to be €192 billion per year. The cost of heart disease and stroke in the United States, includ- ing health care expenditure and lost productivity from deaths and disability, is projected to be more than $475 billion in 2009 [2]. About 10% of the total EU health expenditure is due to CVD, of which 57% is accounted for by direct health care, 21% by productivity losses due to mortality and morbidity and 22% by the informal care of individuals with CVD. CHD and stroke are each responsible for about one fifth of the costs of CVD, although stroke causes less productivity loss due to mortality, but has a higher cost for informal care (Table 1).
Despite the grim statistics outlined above, it is increasingly clear that the risk of CVD can be significantly reduced by relatively easily achievable lifestyle changes, such as switching to a healthier diet, higher in fruit, vegetable and fiber and lower in fat and digestible carbohydrate, increasing exercise and stopping smoking [3]. Postprandial lipemia is now recognised as a risk factor for atherosclerosis, a major cause of CVD (see below), and strong evidence indicates that it is influenced not only by the amount and type of fat consumed, but also by other lifestyle factors such dietary intake of protein, fibre and micronutrients as well as by exercise, alcohol consumption and smoking [4,5] . Since postprandial hyperlipidemia is a feature of increasingly common diseases associated with the Western lifestyle which increase CVD risk, including obesity, insulin resistance and diabetes [4], the condition may represent a link between current lifestyle habits and the rapidly rising incidence of these diseases. This article reviews current knowledge concerning the influence of lifestyle choices on postprandial lipemia and the potential benefits of its routine clinical measurement, and suggests some guidelines for a widely applicable routine test.
POSTPRANDIAL HYPERLIPIDEMIA AS A RISK FACTOR FOR ATHEROSCLEROSIS
Postprandial lipemia refers to the transient increase in blood lipids, particularly triacylglycerols (TG) which occurs after a meal containing fat [5]. It has been estimated, however, that individuals consuming a typical Western spend approximately 18 hours per day in this state [6]. Lipids from the diet, including macronutrients such as fats and micronutrients such as lipid soluble antioxidants, are absorbed in the intestine and packaged into chylomicrons (CM) inside intestinal cells. These large, TG-rich lipoproteins are then secreted into lymph and pass into the blood via the thoracic duct. Here, they are rapidly lipolyzed by lipoprotein lipase (LPL) in extrahepatic capillary beds, forming smaller, but still TG-rich, chylomicron remnants (CMR) which deliver the remaining TG and the other dietary lipids to the liver [7,8]. Once inside the liver, the fatty acids derived from the TG may be used to obtain energy or for the synthesis of complex lipids such as phospholipids. Alternatively, however, they may be re-synthesized into TG, packaged together with cholesterol and phospholipid into very low density lipoprotein (VLDL) and returned to the blood [9]. Since hepatic VLDL secretion is increased postprandially [10], postprandial lipemia is caused by raised levels of CM, CMR and VLDL in the blood. These lipoprotein classes cannot be easily separated from blood samples taken postprandially and are collectively referred to as triglyceride-rich lipoproteins (TRLs).
Thirty years ago, it was first proposed by Zilversmit [11] that postprandial lipemia may play a role in atherogenesis, and there is now a great deal of evidence from epidemiological, clinical and experimental studies to support the idea that postprandial hyperlipidemia is an important risk factor for atherosclerosis and the progression of cardiovascular disease [12-15]. Nakajima and colleagues have reported that the majority of sudden cardiac deaths in a Japanese cohort of patients were associated with postprandial hyperlipidemia [16] and that plasma remnant lipoprotein levels were the major pathological factor involved [17]. Postprandial hyperlipidemia or raised plasma remnant lipoprotein levels have also been linked with increased carotid artery intima thickness in humans [18, 19] and in a rabbit model of hereditary postprandial hypertriglyceridemia [20]. Furthermore, delayed clearance of TRLs has been linked with atherosclerosis progression in a number of studies [12, 21-23] and in two recent large scale prospective trials, non fasting plasma TG levels were found to be related to the incidence of cardiovascular events independently of other risk factors [24, 25].
Current evidence suggests that postprandial lipoproteins may be involved in promoting atherosclerosis both directly, via an influence on events in the vasculature, and indirectly, by causing a more atherogenic lipoprotein profile when their levels are abnormally raised [12, 14, 26]. Atherosclerosis is initiated by dysfunction of the vascular endothelium followed by the formation of macrophage foam cells, generated by the scavenging of lipids from plasma lipoproteins. Accumulation of foam cells and the proliferation of vascular smooth muscle cells (VSMCs) then causes the appearance of fatty streaks, the first visible lesions in the vessel wall [27]. CMR have been shown to penetrate the artery wall and to be retained within the intima [28-30], and remnant-like lipoproteins have been found in human atherosclerotic plaque [31-33]. CMR and TRLs have also been demonstrated to cause endothelial dysfunction [34-38], macrophage foam cell formation [34, 39] and the proliferation of VSMCs [34]. Other pro-inflammatory and potentially atherogenic effects of postprandial lipoproteins include activation of leukocytes, promoting adhesion to the endothelium and invasion of the artery wall [12, 40]. In addition, postprandial lipemia causes pro-thrombotic effects such as increasing coagulant factor VII [41] and plasminogen activator inhibitor type 1(PAI-1) activity [42] and increasing platelet activity [14, 43] and aggregation [44].
Small dense low density lipoprotein (LDL) are thought to be more atherogenic that larger LDL particles because they enter the vessel wall more easily, are more prone to oxidative modification, bind more tightly to the arterial wall and are cleared more slowly [45, 46]. The composition of TRLs is metabolically linked to that of LDL, with plasma TG levels being the main factor influencing LDL particle size [47], and it has been demonstrated that postprandial hyperlipidemia is associated with an increase in the proportion of small, dense LDL [46, 48-50]. In addition, prolonged lipemia leads to increased opportunity for the transfer of core lipids between TRLs and high density lipoprotein (HDL), leading to a reduction in HDL cholesterol levels [14]. This combination of high plasma TG concentrations, increased small dense LDL and low HDL is known as the lipid triad or atherogenic lipoprotein profile [51].
Postprandial lipemia is modulated by many lifestyle factors, including diet, exercise, drinking and smoking, and postprandial hyperlipidemia is a feature of diseases linked to the Western lifestyle, including obesity, insulin resistance and diabetes [4]. Improvements in lifestyle, therefore, should be the first target to control postprandial hyperlipidemia and to reduce the associated risk of cardiovascular disease.
LIFESTYLE AND POSTPRANDIAL LIPEMIA
Although postprandial lipemia is a transient effect which occurs after a fat-containing meal, Western dietary habits of consuming three meals day, often with snacking in between, means that plasma TG levels remain elevated and continually fluctuate in a dynamic, non steady state throughout the day [4, 5]. Clearly, the changes in lipemia will be highly variable from individual to individual depending on the frequency of food intake and the fat (amount and type) composition of the meals. Perhaps less obviously, however, there are a number other modifiable diet and lifestyle factors which play a modulatory role, including the carbohydrate (digestible and fibre), protein and micronutrient content of the food, long term dietary habits, alcohol intake, smoking and the amount of exercise taken, as well as non modifiable factors such as age, gender and genetics [4, 5, 52]. Lifestyle factors affecting postprandial lipemia are summarised in Fig. (1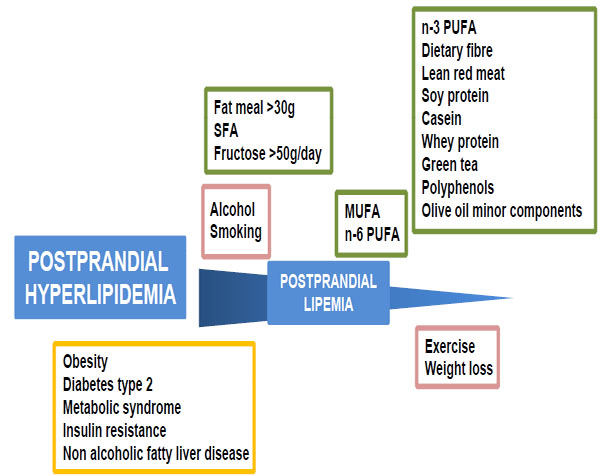 ).
).
 |
Fig. (1) An example screenshot of the virtual lecturer with facial expressions platform (VLFEP). |
Diet
Fat meals containing > 30g fat have been shown to cause postprandial lipemia, with dose dependence up to about 80g [4, 52, 53]. Comparing this to the average content of 20-40g fat in Western style meals and a typical dietary habit of 3-4 meals/day leads to the conclusion that postprandial triglyceridemia commonly lasts for 18h/day in the population of developed countries [6].
Acute studies testing the effects of single meals supplemented with different types of fats have shown that n-3 polyunsaturated fatty acids (PUFA) from fish oil attenuate the postprandial rise in blood TG levels [4, 52, 54] compared to other types of fat, and the saturated fatty acid (SFA) stearic acid found in animal fat has also been reported to have this effect [55-57]. In addition, changes in TRL particle size and number and lipid and apolipoprotein (apo) composition depending on the type of fat in the meal have been demonstrated, with SFA causing the most pronounced lipemia by these criteria, followed by monounsaturated fatty acids (MUFA) (high in olive oil) and then PUFA [4]. It is clearly important, however, to assess the effects of the consumption of different types of fat in the longer term (ie over a period of weeks or months) on postprandial lipemia, since sustained changes in dietary habits are likely to be necessary for beneficial effects on health. The effects of dietary supplementation with n-3 PUFAs in reducing fasting plasma TG levels are well established (see [58] for a recent review), and a number of studies have shown that they also reduce postprandial hypertriglyceridemia, both when chronically present in the diet and when given as a supplement for periods from 4 weeks to 6 months, in normal and hyperlipidemic subjects and in male smokers [59-63]. Moreover, the TG lowering effect has been attributed to a decrease in chylomicron and VLDL production [59, 61]. Other types of fatty acids tend to have less marked effects on postprandial lipemia than n-3 PUFA, but, in general, diets containing or supplemented with MUFA and n-6 PUFA as compared to SFA (found in plant oils) have been found to cause a reduced response [4, 52].
The carbohydrate content of the diet is also believed to influence postprandial lipemia (for a review see [64]). Although there is no consistent evidence to suggest that glucose has any effect, dietary fructose, when given both as the monosaccharide and in the disaccharide, sucrose (sugar), has been shown to enhance the response in a number of studies [4, 64]. A recent meta-analysis covering studies published between 1966 and 2006, however, concluded that there was no significant effect on postprandial blood triacylglycerol levels at doses below 50g/day [65]. Foods high in starch which are common in the Western diet, such as white bread and pasta, do not appear to increase postprandial lipemia, although they do lead to accumulation of CM in the late stages of the postprandial phase [66]. In contrast to the effects of digestible carbohydrate, an increase in the content of indigestible carbohydrate (fiber) of test meals by the addition of oat bran, wheat fibre, wheat germ or psyllium husk has been found to reduce postprandial plasma TG concentrations [4, 64, 67].
There is also some limited evidence to suggest that dietary protein quality influences postprandial lipemia. Diets containing lean red meat, soy protein or casein have been shown to reduce postprandial TRL levels [4, 52] and Mortensen et al. [68] have reported that test meals containing whey protein are more effective in improving the lipemic response than those supplemented with casein, gluten or cod protein in patients with type 2 diabetes.
In addition to macronutrients such as fats, carbohydrate and protein, fruit and vegetables contain micronutrients, bioactive compounds such as vitamins, carotenoids, plant sterols and polyphenols, which are believed to contribute to the protective effective against heart disease of diets rich in these foods and of the intake of beverages such as tea and red wine [69]. Consumption of green tea has been found to reduce postprandial lipemia in hypercholesterolemic subjects, and this effect has been attributed to its polyphenol content [70]. Other studies have suggested that the minor constituents of olive oil may reduce the TG content of TRLs postprandially [71], but plant stanols (derivatives of plant sterols) were found to have no effect on postprandial triglyceridemia in patients on lipid lowering therapy [72].
Alcohol, Smoking and Exercise
Although mild to moderate alcohol intake (equivalent to 0.5 -1 or 1-2 drinks/day for women and men, respectively) is thought to be cardioprotective [73], addition of alcohol to a test meal either as ethanol or red wine has been found to result in a pronounced rise in postprandial lipemia [4, 74], while dealcoholized red wine had no effect [74]. The beneficial effects of moderate alcohol consumption, therefore, are not related to changes in postprandial lipemia. Chronic smoking is also associated with increased postprandial triglyceridemia [4], and this has been attributed to impaired clearance of CM and CMR [75]. From a study including >600 subjects with or without atherosclerosis, Sharrett et al. [6] concluded that, in addition to diet, smoking and alcohol consumption are good predictors of postprandial lipid levels.
The beneficial effects of exercise in reducing postprandial lipemia have been established in a large number of studies [4, 76-79]. Low and moderate volume exercise prior to a fat meal have been shown to decrease the response [76, 78] (although low volume was not effective in smokers [80]), and Singhal et al. [77] reported equally beneficial effects with moderate and high bouts. In addition, a recent review of 16 studies concluded that accumulated exercise (ie in short bouts) may be as effective as continuous exercise in this respect [79]. Moreover, exercise combined with dietary n-3 PUFA supplementation appears to have a synergistic effect in active individuals [4]. The reduction in postprandial lipemia caused by exercise is believed to be due to increased clearance of TRLs which is at least partially mediated by an increase in LPL activity [4].
POSTPRANDIAL LIPEMIA AND METABOLIC DISEASES
Postprandial hyperlipidemia is a feature of common, often inter-related, metabolic diseases, including obesity, insulin resistance, the metabolic syndrome (a combination of obesity, hypertension, insulin resistance/diabetes), non alcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus [12, 34, 81] (Fig. 1 ). The rapid increase in the incidence of these diseases in the last three decades, not only in Western Countries but also in the developing world, has reached epidemic proportions. It has been suggested that diabetes is one of the main threats to health in the 21st century, and it has been estimated that there will be 300 million people worldwide with the disease by 2025 [82]. This explosive rise in metabolic disease is linked to the modern, low exercise lifestyle with a diet rich in highly processed, high fat food and relatively low in fruit, vegetables and fibre [82].
). The rapid increase in the incidence of these diseases in the last three decades, not only in Western Countries but also in the developing world, has reached epidemic proportions. It has been suggested that diabetes is one of the main threats to health in the 21st century, and it has been estimated that there will be 300 million people worldwide with the disease by 2025 [82]. This explosive rise in metabolic disease is linked to the modern, low exercise lifestyle with a diet rich in highly processed, high fat food and relatively low in fruit, vegetables and fibre [82].
Atherosclerosis is the main cause of the increased morbidity and mortality seen in obese patients and is a major cause of death in diabetic patients, and disturbances in postprandial lipid metabolism are particularly implicated in the increased risk [39]. Insulin resistance, a condition commonly found across the different metabolic diseases, is believed to be the primary contributory factor to the postprandial hyperlipidemia [83]. The dyslipidemia is caused by the accumulation of TRLs in the blood due to delayed clearance of CM and CMR coupled with overproduction of these lipoproteins by the intestine and of VLDL by the liver [81, 83, 84].
Factors involved in the excess production of CM in intestinal cells in insulin resistance are thought to include; increased free fatty acid flux into the intestine due to lack of suppression of lipolysis by insulin in adipose tissue; decreased intestinal insulin signaling; and raised activity of the microsomal triglyceride transfer protein (MTP), which is required for CM assembly [84, 85]. The expression of Niemann Pick C-like 1 protein, which regulates intestinal cholesterol absorption, has also been reported to be increased in a rat model of type 2 diabetes, while at the same time there was a decrease in expression of the ATP-binding cassette transporters (ABC) G5 and G8, which export cholesterol back into the lumen of the intestine [86]. In addition, it has been suggested that alterations in the production or action of glucagon-like peptide-1 and -2, which have been implicated in the regulation of CM formation, may also play a role [84]. The delayed clearance of the intestinally derived lipoproteins is believed to be caused by a reduction in the activity of LPL and hepatic lipase, increased competition for lipolysis by LPL because of the rise in VLDL levels, and decreased uptake by the liver due to lower concentrations of apoE in the particles, increased competition for receptors from VLDL and impairment by hyperglycemia [39, 81, 83, 87].
Insulin resistance is also associated with the overproduction of large VLDL by the liver, and this effect has been shown to be due to disturbances in the assembly of the particles within the hepatocyte [88]. Current evidence indicates that VLDL assembly occurs in the rough endoplasmic reticulum (ER) in two steps. Firstly, apoB and TG are brought together in the membrane of the rough ER by a process enhanced by MTP, and further lipidation leads to the formation of a TG poor VLDL particle which may either be secreted, or further lipidated in the second step to form the larger TG-rich VLDLs [88-90]. The TG for the second lipidation step is translocated into the ER lumen by MTP to contribute to VLDL assembly, but may also be returned to the cytosol in a pathway promoted by insulin [89]. In addition, insulin inhibits the expression of MTP, and has also been reported to inhibit the activity of the transcription factor Foxa2, which increases MTP activity [88]. Thus, in normal conditions, insulin down-regulates secretion of the larger VLDL by decreasing the supply of TG for the particles via down regulation MTP activity and diversion of TG to the cytosol, but in insulin resistance these mechanisms fail and there is overproduction of TG-rich particles [88, 89, 91]. In addition to the effects of insulin, it has been suggested that hyperglycemia may play a role in the regulation of hepatic VLDL secretion. High blood glucose levels have been shown to down regulate the secretion of TG in VLDL in normal rats via hypothalamic glucose-sensing mechanisms which act to decrease the activity of stearoyl CoA desaturase-1, an enzyme which promotes VLDL secretion, and to alter a late step in the particle assembly process [88, 92]. This effect, however, was not observed in animals with diet-induced obesity [92], thus it may be a contributory factor in the enhanced production of VLDL in metabolic diseases.
The overproduction of large TG-rich VLDL in insulin resistance and type 2 diabetes is linked to the other dyslipidemias of the atherogenic lipid triad, high levels of small dense LDL and decreased HDL concentrations [51]. The larger particles facilitate the transfer of TG from VLDL to LDL by cholesteryl ester transfer protein (CETP), forming TG-rich LDL which are then preferentially lipolyzed by hepatic lipase to produce the small dense LDL. A similar process involving CETP and hepatic lipase also leads to the formation of small dense HDL which are catabolized at a faster rate, causing plasma HDL levels to fall [88].
The accumulation of TRLs in the circulation in metabolic diseases such as, obesity, insulin resistance and type 2 diabetes provides an increased opportunity for interaction with the artery wall and the promotion of a series of events which lead to the initiation of atherosclerotic lesions. Remnant lipoproteins have been shown to penetrate the vessel wall as efficiently as LDL and to cause foam cell formation [34]. In addition, however, evidence suggests that they may up-regulate the expression of adhesion molecules on endothelial cells and activate leukocytes, thus facilitating their entry into the artery wall, where they differentiate into macrophages eventually become foam cells [12, 40]. TRLs from hyperlipidemic subjects have been shown to up-regulate the expression of pro-inflammatory genes in human endothelial cells, including those coding for the adhesion molecules, vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) [37, 38], and Castro Cabezas and colleagues have demonstrated that there is an increase in neutrophil numbers after a fat meal, accompanied by an increase in plasma inflammatory cytokines [12] which could contribute to endothelial dysfunction. Leukocytes have also been found to take up lipid from TRLs, and to be activated during postprandial lipemia and by CMR, and both types of white blood cells appear to take up TRLs and become lipid enriched in this period [12, 40].
In addition to the vascular dysfunction caused by the increased residence time of TRLs in the circulation, however, in metabolic disease the situation is exacerbated by adverse effects of insulin resistance on the vasculature. Raised plasma glucose and insulin concentrations together with impaired insulin signaling and increased oxidative stress are believed to cause an imbalance in the release of vasodilators and vasoconstrictors and a loss of endothelial-derived nitric oxide bioavailability, as well as increased blood levels of the pro-inflammatory cytokine tumor necrosis factor α (TNFα), which itself can directly alter signaling via the insulin receptor, and all these effects lead to endothelial dysfunction [82, 93, 94]. Furthermore, increased macrophage apoptosis causing cytokine release in the absence of the normal protective effect of insulin may also be an aggravating factor [95].
As well as the changes in cellular function discussed above, insulin resistance also causes modifications in the arterial intima extracellular matrix that are implicated in the increased risk of atherosclerosis development [96]. The binding of apoB containing lipoproteins, including LDL and TRLs, to glycosaminoglycans (GAGs) in proteoglycans in the extracellular matrix causes retention of the lipoproteins in the vessel wall, and is one of the initiating events in atherogenesis [96, 97]. Insulin resistance and type 2 diabetes have been shown to be associated with an increase in GAG length which leads to increased proteoglycan-lipoprotein binding and thus increased retention of the lipoprotein particles in the intima, and this effect has been attributed to raised circulating levels transforming growth factor-β (TGF-β) [96]. Postprandial lipemia may be of particular importance in this mechanism, since a number of studies have suggested that apoB48, found in CM and CMR, has a greater affinity for proteoglycan binding than apoB100, which is found in LDL [96]. Work by Proctor and colleagues has indicated that CMR are co-localized with the proteoglycan, biglycan, in the sub- endothelium of the artery wall in vivo, and although they found no difference in the binding affinity of human biglycan for CM/CMR as compared to LDL in experiments in vitro, the results suggest that the mass of cholesterol bound in CMR is greater than that in LDL [96].
Another possible link between metabolic diseases and postprandial lipemia is adipose tissue. As well as providing a store of energy for the body in the form of TG, adipose tissue actively secretes a number of hormones, including adipokines such as leptin and adiponectin and acylation stimulating protein (ASP) (also called C3adesArg), which is produced on activation of the alternate complement system [98]. Low serum levels of adiponectin have been found to be associated with increased postprandial lipemia and impaired TRL metabolism, and since they correlate with the accumulation of fat in the liver in NAFLD and with the severity of liver histology in non alcoholic steatohepatitis (NASH), this is likely to be due to effects on liver lipid metabolism [99, 100]. Adipocyte production of TNF-α is also raised in insulin resistance, and as well as direct effects on insulin signaling, this leads to down-regulation of genes regulating lipid storage such as LPL and glycerol phosphate dehydrogenase [93]. ASP increases the storage of TG in adipose tissue, and also inhibits TG lipolysis [93, 98]. Plasma levels of fasting ASP have been shown to be positively correlated with postprandial TG concentrations, and are also raised in obesity and insulin resistance, but it is not known whether this indicates ASP resistance or whether it is a compensatory response to postprandial hyperlipidemia [93, 98].
TRANSLATION TO PREVENTION
Because of the increased risk of cardiovascular disease associated with postprandial hyperlipidemia, particularly in conditions such as obesity and type 2 diabetes, it is recommended that it should be treated [101]. Since the modern Western lifestyle is largely responsible for the problem, it seems reasonable to begin by changing in factors such as diet and exercise. It has been suggested that humans are genetically adapted to a hunter-gatherer lifestyle [73, 83] and that diets such as the traditional Mediterranean diet are healthy because they match the ancestral eating patterns much more closely than the modern diet. Significant reductions in postprandial lipemia can be achieved by switching to a diet rich in fresh, unprocessed or minimally processed fruit, nuts and vegetables and containing moderate amounts of MUFA (eg from olive oil) and n-3 PUFAs (eg from oily fish) and some high quality protein (eg lean meat), but low in processed carbohydrate and saturated fats [73]. Regular consumption of nuts (5 times per week or more) has been reported to reduce diabetes risk by 20-50%, while restriction of the intake of refined carbohydrates such as white bread has been shown to reduce postprandial hypertriglyceridemia and intra-abdominal fat in insulin resistant subjects, and replacing it with MUFA from nuts or olive oil (which may be achieved for example, by replacing sugary snacks with nuts) has been found to have similar beneficial effects [73]. One reason for this is that remnant clearance seems to be optimized when the particles are enriched in TG with MUFA in the glycerol 2-position [102]. Dietary n-3 PUFAs are known to lower postprandial plasma TG levels in healthy individuals, and experiments with animal models of insulin resistance have shown that they reduce postprandial lipemia and the intestinal production of apoB48 containing lipoproteins [103, 104].
In addition to dietary changes, weight loss, increased physical activity and cessation of smoking can also help to correct the postprandial hyperlipidemia associated with metabolic diseases [4, 6, 73, 75-80]. Weight loss of >5%, preferably via a diet that restricts the intake of processed carbohydrates and saturated fats, reduces postprandial lipemia and the associated inflammatory effects [74], and Volek et al. [105] have reported that a carbohydrate-restricted as compared to a low fat hypocaloric diet is more effective in subjects with the metabolic syndrome. In addition, exercise improves insulin sensitivity and lowers blood postprandial TG levels, and two recent studies have concluded that exercise of moderate intensity before a fat meal attenuates postprandial hypertriglyceridemia in insulin resistant males [106, 107]. These findings suggest that if the main meal of the day were routinely preceded by a period of exercise, chylomicron clearance should be optimized. Thus, it may be beneficial if the main meal were taken at lunchtime with only a light evening meal, instead of the main meal at the end of the day followed by physical inactivity, which is the current pattern in most Western societies. Intervention studies could easily test these hypotheses.
There are a number of possible pharmacological approaches which may be effective in reducing postprandial hyperlipidemia in obese and diabetic patients, including statins, fibric acid derivatives, nicotinic acid, and oral omega-3 fatty acid supplements [81, 101]. However, the changes in diet indicated above, coupled with weight loss and increased exercise, can be remarkably effective in producing immediate improvements [73] and pharmacological intervention is warranted only in the event that the desired reduction is not achieved after 3 to 6 months of the lifestyle changes [81, 101].
BIOMARKERS
Alterations in fasting plasma lipid lipoprotein concentrations are known to influence the risk of CVD [108]. In the last decades it has been established that total plasma cholesterol and LDL cholesterol are the best biomarkers of plasma lipemia for evaluation of CVD risk. Using LDL levels, the prevention of CVD is mainly based on a pharmaceutical approach (use of statins), which has proved to be extremely effective [108]. However, LDL elevation is absent in many patients with atherosclerosis and about 1/3 of heart attacks remain unpredicted by this parameter. Furthermore, in fasting normolipidemic subjects [21, 109] increased CVD risk is associated with an exaggerated postprandial lipemic response.
A second factor in CVD prevention is based on changes in lifestyle, and there is a growing consensus that plasma TG levels may be a better reflection of aspects of lipid metabolism that are not revealed by the plasma cholesterol profile. Dietary change, body weight control, increased physical activity, sleeping modality and cessation of smoking are the most common interventations to prevent and reduce cardiovascular risk and related complications associated with metabolic diseases [4,6,73, 75-81]. In the last decades most of these recommendations have developed empirically, mainly on the base of epidemiological studies, without clear understanding of the underlying mechanisms. Although the importance of postprandial metabolism has been debated for a long time, nowadays, as reported above, sufficient evidence exist that atherosclerosis can develop as a consequence of disordered CM metabolism, and that this is probably the most common etiology, as it occurs in patients with CHD and other associated conditions such as hypertension, familiar hypercholesterolemia, obesity, metabolic syndrome, NASH, and renal disease. Acceptance of the role of CM in atherogenesis aids the understanding of the mechanisms underlying empirical risk-reduction strategies, and provides impetus for methodological research to find reliable routine biomarkers of postprandial hyperlipidemia.
Many aspects of studies on CM and CMR metabolism and related topics have been recently reviewed (see Atherosclerosis Supplements Vol. 9, 2008) as well the necessity for a prospective study to investigate the role of postprandial lipid metabolism in CAD [110]. Thus, a major aim of this article is to translate the present knowledge on postprandial lipemia into recommendations for common, more uniform guidelines for a prototype of a routine test for the assessment of this parameter.
Since CM, CMR, VLDL and their remnants all contribute to postprandial TG levels, a deeper understanding of postprandial metabolism in TG-rich lipoproteins necessitates a combination of several methodological approaches [110-112]. However, from the clinical point of view, it is relevant that an elevated concentration of TG is the main lipid change found in plasma postprandially, and the magnitude of the response and the slow return to the fasting levels, which mainly reflect persistence of CMR in the circulation, is associated with increased risk of CAD [113].
In the future, when accurate and simple methods to separate remnants from other lipoproteins may be available, determination of remnant-cholesterol will be an interesting alternative [24], but, so far, methodological considerations suggest that the most wide-applicable and reliable approaches for the bio-clinical routine laboratory are the determination of plasma TG and apoB48 [114] after consumption of a standard test meal. The advantage of measuring apoB-48 is that this parameter indicates the number of lipoprotein particles of intestinal origin in postprandial plasma, which may be of critical importance in assessing the risk of CVD. It is noteworthy that, in terms of cost/benefit, often a deciding factor for the realization of large non population-addressed studies, apoB48 determination is far more expensive and less standardized than TG analysis, which thus remains the more appropriate marker to study the postprandial phase in the routine laboratory. In fact, although correlation between fasting and nonfasting TG concentrations is high, evidence suggests that postprandial TG may be a more potent predictor of risk, indicating that the variability in postprandial TG concentration may give relevant information about an individual’s metabolism [24,115]. However, although Zilversmit [11] first hypothesized the need to investigate the lipid profile after the administration of an oral test fat meal about 30 years ago, and Karpe proposed a standardized methodology in 1997 [116], the measurement of postprandial TG is still not standard clinical practice, and there remains a lack of consensus on the methodology that should be used to to study postprandial lipemia. There are a number of reasons for this. Firstly, acceptance of the concept of the superiority of the predictive role of non fasting TG over the routine TG fasting determination has been slow. Secondly, questions about the reliability of plasma TG determination, and thirdly a lack of general agreement on guidelines for a standardized postprandial test (meal, sampling time, result calculation, reference values) have also contributed. As the variability of the postprandial response is very high, one obvious obstacle is difficulty in interpretation of the results due to the lack of reference interval values. The last point may be overcome only by wider implementation of postprandial tests and the definition of reference values on the base of geographic background diet differences.
In the past, conflicting conclusions on the role of fasting TG in the evaluation of CAD risk have also been related to analytical considerations. The routine use of fasting TG measurements derived mainly from the necessity of clinicians to use this data to calculate LDL-cholesterol using the Friedewald equation. In comparison to the largely unexplored reference values in the postprandial phase, the consolidated common utilization of this assay has contributed to a better definition of fasting TG plasma reference intervals. However, the variability remains high, mainly because TG is a difficult metabolite to analyze. TG consist of 3 fatty acids esterified to one glycerol molecule. Most of the common methods determine TG in terms of the glycerol content, employing lipase enzymes to remove the fatty acids [117]. TG, however, are a heterogeneous mixture of molecular species with different fatty acid compositions and consequently, different affinities for the lipases used for their assay. This contributes to the large fluctuation in TG determinations over time which have been estimated to have a coefficient of variation (CV) of biological variability averaging about 23% and ranging up to 40% [118]. In fact, in individual patient management, single fasting TG measurements are unreliable, with intra-individual CVs up to 60% [119]. Thus, a long fasting interval (10-12h) before the analysis of post prandial lipemia is generally recommended to lower the within-subject CV variability. However, as this is influenced by the background diet, the differences cannot be completely abolished. In future, the use of assays that remove or correct for the presence of free glycerol in the sample, for example, by depleting glycerol in a preliminary reaction by the addition of glycerol kinase and catalase, should be encouraged.
Although the first meta-analysis based on Western populations showing that fasting TG is an independent risk factor for CVD, even in the range previously considered to be normal (below 2.0 mmol/L) [120], was published in 1996 [121], controversy still exists regarding the clinical usefulness of fasting triglycerides as an independent predictor of risk [122], because adjustment for other covariates, in particular HDL-cholesterol, markedly decreases both the magnitude and significance of observed epidemiologic effects [46, 123]. However, in 2007, two very large independent prospective fully corrected analyses that compared the predictive value of nonfasting TG towards fasting TG reached the conclusion that nonfasting TG is superior to conventional fasting TG in predicting future cardiovascular events after multifactorial adjustment for other cardiovascular risk factors [24,25]. A prospective study of 26,509 women with a follow-up of a median of 11.4 years showed that nonfasting TG is associated with the incidence of cardiovascular events, independent of traditional cardiac risk factors, levels of other lipids, and markers of insulin resistance [ 24,25]. In the second prospective cohort study involving the general population of Copenhagen followed for 11 years, elevated nonfasting TG levels were associated with increased risk of myocardial infarction, ischemic heart disease and death [25]. Observations consistent with this have also been made in other studies [121, 124], including those on pacific-Asian populations [116, 125], and similar conclusions are reported in the first prospective comparison of the association between the metabolism profile of a panel of lipids and CVD depending on the time of last meal [126]. Moreover, van Oostrom et al. [112] have shown that the area under the curve (AUC)-TG, resulting from daylong measurement of capillary blood in free-living subjects, is a better predictor of CVD than fasting lipid parameters such as LDL-cholesterol.
A further potential advantage of moving from fasting to nonfasting TG derives from the observation that postprandial TG levels reflect liver fat content [100, 127]. Matikainen et al. [100] have reported that hepatic fat accumulation is closely correlated to postprandial lipids, and predicts the responses of CM and large VLDL better than measures of glucose metabolism or body adiposity. This growing evidence for the consistency of the predictive role of postprandial TG is increasing the need to perform wider studies involving postprandial metabolism evaluation [110, 111, 128]. It should be noted, however, that separately from the assessment of CVD risk, determination of plasma TG is used routinely for the diagnosis of pancreatitis, and for this purpose, the fasting condition is irrelevant.
BIOMARKERS FOR POSTPRANDIAL RESPONSE
The acute metabolic response to a fat meal and nutrients is influenced by several factors. However, on the basis of published reports, there are a number of concepts that may be taken as a base for the standardization of conditions for the determination of nonfasting TG.
Preliminary
Our aim in this article is to propose a wider use of the routine determination of nonfasting TG as a marker for CVD risk. A complicating factor, however, is that it is practically impossible to avoid the influence of background life style. A general recommendation, therefore, should be to avoid any significant change of the usual diet and habits the day before the test, including alcohol and smoking habits. Extreme physical activity and food excess should also be avoided.
Fat Meal
A small amount of fat (<15g) does not alter plasma lipoprotein levels, but the ingestion of about 30-40g induces the secretion of CM with a composition which is highly dependent on quantity and type of fat, energy intake, carbohydrate, protein, and other dietary components of the meal [129]. The quantity and quality of the test meal can vary the CM response to a fat challenge by more than 50%. Several fat meals, varying in size and composition [reviewed in 111 and 122] have been used to compare postprandial lipemia in subjects at different disease-risk or to study the response to lipids or other specific foodstuffs or to habitual dietary regimen. Thus, it is not surprising that findings differ between groups.
Size of the meal is a crucial point. In recent literature, no general tendency can be observed in the methods used to calculate the meal size. The administration of a meal with the same components and energy content irrespective of body weights and therefore of blood volumes has been used more often than the approach of sizing the test meal according to the unit dose per Kg body weight or per body surface area [23, 130, 131]. As in adults the main variable of body weight is fat mass, in obese subjects calculations based on body weight can cause an overestimation of lipid load. So far, the interesting idea of using total adipose tissue mass [111] to determine the meal size has not been taken up. In view of the lack of a definitive answer, in order to standardize postprandial lipemia evaluation for ease of clinical use, the simpler approach would be to size the fat load according to the body surface area.
Habitual dietary habits are a strong determinant of postprandial metabolic capacity, and the type of predominant fat in the diet varies in different countries and cultures [54, 131, 132]. Thus, it may be not important to specify a particular type of fat for use in clinical practice, but rather to use typical components of the region and, possibly, to align the timing of the test with normal habits. This would be the best solution for routine assessment. However, given the important effect that the degree of fatty acid saturation present in dietary TG have on postprandial lipemia, it is desirable that test meals used for this type of study have a similar degree of saturation level in all parts of the world.
The first type of test meals used were based on dairy-fat based snacks [111, 133, 134]. However, the need for meals that stimulate the physiological functions of digestive organs, as well as the release of insulin necessary for dietary TG processing, suggest that mixed liquid-solid meals, which favor normal gastric emptying and also contain carbohydrate and protein, are a better choice. At low and very high doses of dietary lipids there is no clear dose dependence of postprandial hypertriglyceridemia on the fat load. However, a dose-dependent increase has been reported with moderate intake of between 30-60g of fat [129]. Based on this, a mixed meal, having a total energy content of 650-950Kcal, with 50-65% from fats, a significant proportion (25-40%) from carbohydrate and the remainder from protein, is used most commonly. Another reason why the previous meals containing dairy fats are no longer considered optimal is that they contain significant amounts of short and medium-chain fatty acids that enter the blood directly via the portal route, lowering the triglyceridemic response linked to CM secretion [55, 133, 134]. As the test is also appropriate for at risk-subjects or patients, the standardized fat meal should have a moderately high energy content of about 600-800kcal.
The carbohydrate content (50-100g) of the meal is important to ensure the effective release of insulin. However, the nature as well as the amount of carbohydrate can influence postprandial lipid responses [135]. Meals containing highly digestible starch food seem preferable to those containing glucose and fructose, and those containing slowly digestible carbohydrate that induces low glycemic and insulinemic responses have been reported to decrease postprandial hyperlipidemia in obese subjects [66]. Thus, it is important to determine the effects of the starch source in the meal on plasma glucose and insulin during such studies performed either in normal or at risk-subjects or in patients [111].
Concerning the effect of cholesterol, it has been shown that a chronic diet enriched with dietary cholesterol does not elicit noticeable changes in postprandial lipemia [136]. Prediction of the effect of the meal cholesterol content on postprandial triglyceridemia, however, is complicated because of the prolonged multi-phase duration of its absorption [132]. The data currently available suggests that more than 250mg of cholesterol in the test meal enhances the postprandial increase in CM-TG [129] . Therefore, a standardized test meal for biochemical evaluation of postprandial lipemia should include a defined low to moderate dose of cholesterol (200-250mg).
Taking into account the factors discussed above, we propose a possible breakfast test meal developed on the basis of Mediterranean (Italian) habits and food ingredients.
Breakfast Cake
Flour: 300 g
Sugar: 200 g
Olive oil: 220 g
Dry almonds: 300 g
Eggs: 5
Baker’s yeast: 65 g
Total energy content 6,456 kcal
Consumption of 130 g of this cake with a little Italian bitter coffee provides an 807 kcal breakfast, of which 475 kcal (59%) is fat. This meal has several advantages. Change of the source of fat (type of oil, margarine butter) can be made depending on dietary habits in different geographical regions, while most of the other ingredients are used worldwide in cakes and pastry.
Schedule Time
Studies on the effects of the time of day of consumption of a fat meal on the postprandial lipemic response have shown that there is a clear circadian variation [137-139]. Compared with breakfast time, the same meal consumed at lunch elicited a significantly smaller rise in plasma TG. In addition, compared with a morning or mid-day test in the same patients, a nocturnal test showed a later or increased peak TG concentration and a delayed return to baseline concentrations. The nocturnal test was better tolerated in the patients because it did not disrupt their normal activity and eating patterns, as morning tests often do. Some other studies have been conducted overnight following an evening meal [54, 111], with standardization of the preceding day’s food intake. Overnight studies allow blood sampling to be continued for 12–14 h and demonstrate that TG return towards ‘fasting’ levels only in the early hours of the morning (0400–0500 hours) after a meal the night before (1900 hours) [137, 139]. Thus, standardization of the time of day at which the postprandial TG test is performed is essential.
For routine clinical purposes, the morning is the most suitable time of the day to perform the test. Common sense suggests, therefore, that both to improve patient acceptance and to incorporate the meal into the usual daily habits, it should be similar to typical regional breakfast. A basal blood sample should be withdrawn from the subject after an overnight fast and a short rest in the laboratory, followed shortly afterwards by consumption of the test meal in a fixed time (15–20 min). Delayed clearance of postprandial TG is the main hallmark of a hyperlipidemic postprandial response. Most of the studies now available agree that most informative TG determinations are those at 3-6 h after the meal [24, 25, 111, 122]. Measurements made at baseline and after 4 h (expected peak) and 8 h postprandially correlate well with data obtained using more frequent time points [140] . Bansal et al [24] showed that in fully adjusted analysis, TG levels measured 2 to 4 h postprandially had the strongest association with cardiovascular events [25]. Thus, based on current evidence, the best recommendation is to take postprandial blood after 4 h. If this is impracticable, for example for subjects who are in full time employment, a longer, rather than shorter time is advisable.
Calculation
Calculation of the results is the final step. Although the ultimate aim is to encourage a more widespread use of the determination of postprandial TG levels to assess CVD risk and to reach a consensus on a single determination of postprandial TG, because of the current lack of availability of reliable reference values, it will be necessary, at least in the preliminary phase, to evaluate individual postprandial responses by determining the incremental postprandial TG concentration in relation to the baseline value. In the past, several methods have been used for calculating and expressing the data obtained during postprandial studies. It is now clear that when several determinations are available, the most correct way is to calculate the AUC generally, by the trapezoidal method. For follow-up, in the case of basal and 4 h determinations, the incremental concentrations obtained by subtracting baseline values from those obtained postprandially (baseline value becomes 0) may represent the best way to represent the relative changes from baseline at the different time points. For the early phases of routine determination of nonfasting TG, both the absolute concentration and the 4 h increment could be useful to the clinician, both to evaluate the postprandial response and the way in which it may be influenced by changes in lifestyle habits or pharmaceutical therapy.
Although reference interval values will need to be evaluated in individual populations under the conditions of the chosen meal, to support the initial studies we report in Table 2 indicative values of postprandial lipemia 4h after consumption of a mixed solid meal [122,140,141]. As reported in the text a number of lifestyle factors and related diseases (diabetes, obesity, smoke, hypertension, etc) may induce alterations in postprandial triglyceridemia, and intermediate values may be of particular interest to evaluate therapeutical efficacy. This table also reports cholesterol levels, however, postprandial cholesterolemia has little, if any, clinical value, and fasting blood cholesterol levels are a better index for diagnostics, therapeutics and disease prevention.
FUTURE
In view of recent studies showing that a single non-fasting plasma TG measurement is a better independent pre-dictor of CVD risk than fasting TG [13, 24, 25], several authors hoped for prognostic prospective studies [110, 111, 122, 128] with standard meals to facilitate a move from fast-ing to nonfasting TG assay as a routine clinical test. How-ever, the translation to clinical routine of a nonfasting TG test for its prognostic value may still be hampered by cost/benefit considerations. On the other hand, current knowledge of CMR metabolism indicates that the fat load test meal is not only of benefit prognostically, but is also important therapeutically. When pathological changes in postprandial TG reflect CMR clearance times, then positive responses to life style/drug interventations should be accompanied by changes in the nonfasting TG response. As several options for lifestyle improvement as well as a number of different type of drugs [142] are available for medical inter-ventation, the option of evaluating changes in CMR metabo-lism by determination of nonfasting TG is fascinating and likely to increase knowledge on the relationship between empirical life style recommendations and postprandial hy-perlipidemia.
The last considerations of this article are about the importance of the topic addressed by this paper in relation to the large social, economic, public health issues, and the translation of our extensive knowledge on the role of nutrition in health into national and international policy [143]. At the population level, a healthy diet and appropriate exercise throughout life can reduce the threat of a global epidemic of chronic diseases [143-145]. Considering the prediction that, if current trends continue, the worldwide incidence of type 2 diabetes will reach 300 million by 2025 [82], there would clearly be a favourable cost/benefit in investing in psychological support to help people to make healthier lifestyle choices. The world-wide aim of National Public Health Systems is to promote the new field of 'public nutrition' as a means to address, in a more efficient, sustainable and ethical manner a population-level approach to the prevention of the major nutrition-related health problems. Greater use of postprandial lipid determination would contribute to the establishment of an evidence-based approach to the implementation and evaluation of empirical interventations which aim to improve the nutrition-related health in the population.
ACKNOWLEDGEMENT
Part of the work discussed was supported by the Ricerca Finalizzata of the Italian Ministry of Health (Ref ISS R26).


