- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Virology Journal
(Discontinued)
ISSN: 1874-3579 ― Volume 15, 2021
Pathogenic Characterization of European Genotype Porcine Reproductive and Respiratory Syndrome Virus Recently Isolated in Mainland China
Sun Ming, Ma Yongying, Liu Bohua, Lu Huiying, Deng Xiaoyu, Liu Qiaorong, Qiao Mingming, Chen Xi, Yang Xinyan, Chen Xizhao*
Abstract
Background:
Porcine reproductive and respiratory syndrome virus (PRRSV) is an important pathogen in pig that causes tremendous economic loss in the global swine industry. PRRSV is divided into the European and North American genotypes, with virulence ranging from apathogenic-moderately virulent to highly pathogenic. The emergence of new highly virulent type 1 strains and coexistence of the two genotypes complicate the differential diagnosis, disease prevention, and control of PRRSV. Although the emergence of a novel type 1 PRRSV strain in mainland China was first confirmed in 2011, there is no information available concerning the pathogenesis of this strain.
Objectives:
We sought to determine the pathogenesis of a newly emerged Chinese type 1 PRRSV strain HLJB1.
Methods:
Pigs were infected with HLJB1 and characterized using serological and histopathological tests.
Results:
HLJB1 infection induced transient chemosis, reddened conjunctiva, skin cyanosis, mild transient pyrexia, dyspnea, and tachypnea between 7 and 13 days post-infection. Gross pneumonic lesions were characterized by multifocal, tan-mottled areas. Lymph nodes and spleen were enlarged. Characteristic microscopic lesions consisted of pulmonary consolidation and alveolar septal thickening with red blood cell infiltration, depletion of splenic lymphocytes, and hyperplasia and activation of macrophage. No pigs infected with HLJB1 died during the experiment.
Conclusion:
Our findings indicate that Chinese type I PRRSV strain HLJB1 caused classic PRRSV-specific lesions. As it caused lower viremia in pigs compared with other classic type 1 isolates, HLJB1 is less virulent than other type I strains.
Article Information
Identifiers and Pagination:
Year: 2017Volume: 11
Issue: Suppl-1, M6
First Page: 83
Last Page: 89
Publisher Id: TOVJ-11-83
DOI: 10.2174/1874357901711010083
Article History:
Received Date: 06/10/2016Revision Received Date: 01/11/2016
Acceptance Date: 06/02/2017
Electronic publication date: 30/06/2017
Collection year: 2017
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the No.68 Beiqing Road, Haidian District, Beijing, China; Tel: +86-10-82898320; Fax: +86-10-82898320; E-mail: chenxizhao@anheal.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 06-10-2016 |
Original Manuscript | Pathogenic Characterization of European Genotype Porcine Reproductive and Respiratory Syndrome Virus Recently Isolated in Mainland China | |
INTRODUCTION
Porcine reproductive and respiratory syndrome virus (PRRSV) is a small, enveloped, positive-stranded RNA virus that belongs to the order Nidovirales, family Arteriviridae, and genus Arterivirus [1Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch Virol 1997; 142: 629-33.]. PRRSV is the causative agent of porcine reproductive and respiratory syndrome (PRRS). PRRS has become one of the most significant and economically important infectious diseases affecting swine worldwide, characterized by reproductive failure in sows and respiratory problems in piglets and growing pigs [2Meulenberg JJ, Hulst MM, de Meijer EJ, et al. Lelystad virus, the causative agent of porcine epidemicabortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology 1993; 192(1): 62-72.
[http://dx.doi.org/10.1006/viro.1993.1008] ]. PRRSV shows a high degree of genetic variation [3Meng XJ, Paul PS, Halbur PG, Lum MA. Phylogenetic analyses of the putative M (ORF-6)-gene and N (ORF-7)-gene of porcine reproductive and respiratory syndrome virus (PRRSV): implication for the existence of 2genotypes of PRRSV in the USA and Europe. Arch Virol 1995; 140: 745-55.
[http://dx.doi.org/10.1007/BF01309962] , 4Nelsen CJ, Murtaugh MP, Faaberg KS. Porcine reproductive and respiratory syndrome virus comparison: divergent evolution on two continents. J Virol 1999; 73: 270-80.] and some antigenic heterogeneity [5Wensvoort G, de Kluyver EP, Luijtze EA, et al. Antigenic comparison of Lelystad virus and swine infertility and respiratory syndrome (SIRS) virus. J Vet Diagn Invest 1992; 4: 134-8.
[http://dx.doi.org/10.1177/104063879200400203] , 6Wieczorek-Krohmer M, Weiland F, Conzelmann K, et al. Porcine reproductive and respiratory syndrome virus (PRRSV): monoclonal antibodies detect common epitopes on two viral proteins of European and U.S. isolates. Vet Microbiol 1996; 51: 257-66.
[http://dx.doi.org/10.1016/0378-1135(96)00047-8] ]. Based on genetic and antigenic characteristics, PRRSV is divided into two distinct genotypes: the European genotype (type I), with the prototype strain Lelystad virus representing viruses predominately originating from Europe; and the North American genotype (type II), with the prototype strain VR-2332 representing viruses predominately originating from North America. Both genotypes share approximately 55–70% nucleotide identity [7Andreyev VG, Wesley RD, Mengeling WL, Vorwald AC, Lager KM. Genetic variation and phylogenetic relationships of 22 porcine reproductive and respiratory syndrome virus (PRRSV) field strains based on sequence analysis of open reading frame 5. Arch Virol 1997; 142(5): 993-1001.
[http://dx.doi.org/10.1007/s007050050134] ]. There is increasing diversity among strains of the two genotypes, which has been attributed to the high error rate inherent in PRRSV replication and recombination between strains [8Murtaugh MP, Stadejek T, Abrahante JE, Lam TT, Leung FC. The ever-expanding diversity of porcine reproductive and respiratory syndrome virus. Virus Res 2010; 154(1-2): 18-30.
[http://dx.doi.org/10.1016/j.virusres.2010.08.015] ]. According to recent reports on PRRSV classification, type I is divided into three subtypes: a pan-European subtype 1 and East European subtypes 2 and 3 [9Stadejek T, Oleksiewicz MB, Scherbakov AV, et al. Definition of subtypes in the European genotype of porcine reproductive and respiratory syndrome virus: nucleocapsid characteristics and geographical distribution in Europe. Arch Virol 2008; 153: 1479-88.
[http://dx.doi.org/10.1007/s00705-008-0146-2] ]. Historically, type 1 was restricted to Europe and type 2 to North America; however, coexistence of the two genotypes has been identified in Europe, North America, and Asia, complicating the differential diagnosis, disease prevention, and control of PRRSV [10Amonsin A, Kedkovid R, Puranaveja S, Wongyanin P, Suradhat S, Thanawongnuwech R. Comparative analysis of complete nucleotide sequence of porcine reproductive and respiratory syndrome virus (PRRSV) isolates in Thailand (US and EU genotypes). Virol J 2009; 6: 143.
[http://dx.doi.org/10.1186/1743-422X-6-143] , 11Balka G. Hornya k, A., Ba lint, A., Kiss, I., Kecskeme ti, S., Bakonyi, T. Rusvai, M. Genetic diversity of porcine reproductive and respiratory syndrome virus strains circulating in Hungarian swine herds. Vet Microbiol 2008; 127: 128-35.
[http://dx.doi.org/10.1016/j.vetmic.2007.08.001] ]. These genotypes cause similar clinical signs but exhibit significantly different virulence potentials, ranging from apathogenic-moderately virulent to highly pathogenic. Type 2 PRRSV strains induce more severe respiratory disease than type 1 PRRSV strains [12Halbur PG, Paul PS, Frey ML, et al. Comparison of the pathogenicity of two USporcine reproductive and respiratory syndrome virus isolates with thatof the Lelystad virus. Vet Pathol 1995; 32: 648-60.
[http://dx.doi.org/10.1177/030098589503200606] -14Van der Linden IF, Voermans JJ, van der Linde-Bril EM, Bianchi AT, Steverink PJ. Virological kinetics and immunological responses to a porcine reproductive and respiratory syndrome virus infection of pigs at different ages. Vaccine 2003; 21: 1952-7.
[http://dx.doi.org/10.1016/S0264-410X(02)00822-8] ].
During the last decade, new highly virulent strains have emerged in Eastern Europe, China, Vietnam, and South Korea, causing more severe clinical signs [15Lyoo KS, Yeom M, Choi JY, Park JH, Yoon SW, Song D. Unusual severe cases of type 1 porcine reproductive and respiratory syndrome virus (PRRSV) infection in conventionally reared pigs in South Korea. BMC Vet Res 2015; 11: 272.
[http://dx.doi.org/10.1186/s12917-015-0584-5] -17Karniychuk UU, Geldhof M, Vanhee M, Van Doorsselaere J, Saveleva TA, Nauwynck HJ. Pathogenesis and antigenic characterization of a new EastEuropean subtype 3 porcine reproductive and respiratory syndromevirus isolate. BMC Vet Res 2010; 6: 30.
[http://dx.doi.org/10.1186/1746-6148-6-30] ]. An initial outbreak of PRRS in mainland China occurred at the end of 1995 and has since become widespread. The current infection rate of Chinese swine herds has risen to 90%, with PRRS now one of the most significant problems for Chinese swine production [18Guo BQ, Chen ZS, Liu WX, Cui YZ. Isolation of PRRSV from aborted fetus with typical clinical PRRS manifestations. Chin J Prev Vet Med 1996; 2: 1-5. [in Chinese].]. Research on PRRSV in China has primarily focused on North American PRRSV isolates, particularly highly pathogenic PRRSV isolates in recent years [19Gao ZQ, Guo X, Yang HC. Genomic characterization of two Chinese isolates of porcine respiratory and reproductive syndrome virus. Arch Virol 2004; 149: 1341-51.
[http://dx.doi.org/10.1007/s00705-004-0292-0] -21Zhou L, Chen S, Zhang J, et al. Molecular variation analysis of porcine reproductive and respiratory syndrome virus in China. Virus Res 2009; 145: 97-105. a
[http://dx.doi.org/10.1016/j.virusres.2009.06.014] ]. In contrast, only a few Chinese type 1 PRRSV sequences have been submitted to the GenBank database with no corresponding research. Therefore, there is a lack of information about the virulence of Chinese type 1 PRRSV strains.
HLJB1 is a newly emerged type 1 PRRSV strain recently isolated from pig in mainland China, and its complete genomic sequence has been determined (GenBank accession no. KT224385.1). Phylogenetic analysis of type 1 PRRSV strains based on the nucleotide sequences of complete ORF5 genes indicated that HLJB1 belongs to a separate phylogenetic branch from other known type 1 PRRSV strains. The aim of this study was to characterize the pathogenicity of recently isolated Chinese type 1 PRRSV strain HLJB1 in pigs after experimental infection.
MATERIALS AND METHODS
Viruses
HLJB1 (passage 5) was propagated in pulmonary alveolar macrophages at a titer of 1×105 TCID50/ml in Dulbecco’s modified Eagle’s medium supplemented with 5% fetal bovine serum, penicillin (100 units/ml), and streptomycin (100 ug/ml).
Animals And Experimental Design
At 8 weeks of age, nine conventional pigs from a PRRSV-negative herd were purchased from a PRRSV-free commercial farm. All pigs were PRRSV-negative according to routine serology and real-time PCR (RT-PCR) analysis prior to delivery and on arrival. All pigs were individually housed throughout the experiment in an environmentally controlled building within pens over completely slatted floors. The pigs were randomly assigned to two groups and kept for 7 days to allow adaptation to the new conditions. The pigs in group 1 (n = 7) were inoculated oronasally with 105 TCID50/pig HLJB1 in 2 ml of phosphate-buffered saline (PBS). The pigs in group 2 (n = 2) were inoculated oronasally with 2 ml of PBS and served as negative controls. Clinical signs (body temperature, respiratory disorders, and general signs such as appetite and behavior) were monitored daily in both groups starting from 6 days pre-inoculation until the day of death or euthanasia.
Serological Tests
Blood samples from HLJB1-inoculated pigs were collected at 0, 7, 14, 21, and 30 days post-infection (dpi). Serum samples were tested using a commercially available PRRSV enzyme-linked immunosorbent assay (ELISA; HerdChek PRRS 2XR, IDEXX Inc., USA). Serum samples were considered positive for PRRSV antibody if the S/P ratio was greater than 0.4 according to the manufacturer’s instructions.
Gross And Histopathological Tests
At 10, 15, and 21 dpi, three pigs were euthanized and lungs, tonsils, spleen, and inguinal lymph node samples were collected. Two samples from each location were collected. One sample was frozen and stored at -70°C, while the other was fixed in 4% formaldehyde solution and further processed for histopathology using hematoxylin and eosin staining.
Quantification of PRRSV RNA
The QIAamp Viral RNA Mini kit (QIAGEN GmbH, D-40724 Hilden) was used to obtain viral RNA from serum samples. A 140-ul volume of serum was used for the extraction. RT-PCR for type 1 PRRSV was performed to quantify PRRSV genomic cDNA copy numbers, as previously described [22Wasilk A, Callahan JD, Christopher-Hennings J, et al. Detection of U.S., Lelystad, and European-like porcine reproductive and respiratory syndrome viruses and relative quantitation in boar semen and serum samples by real-time PCR. J Clin Microbiol 2004; 42: 4453-61.
[http://dx.doi.org/10.1128/JCM.42.10.4453-4461.2004] ].
RESULTS
Clinical Signs
Experimental infection of Chinese type 1 PRRSV strain HLJB1 in pig induced fever, transient chemosis, reddened conjunctiva, skin cyanosis, mild transient pyrexia, dyspnea, and tachypnea. At 2 dpi, six of seven pigs already had fever (body temperatures ≥40°C) that lasted for more than 3 days (Fig. 1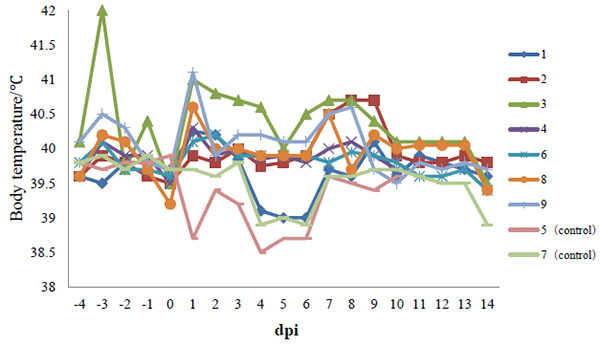 ). Anorexia and depression were seen in one pig from 11 to 13 dpi. All seven pigs exhibited respiratory symptoms to varying degrees; wheezing was observed in one pig at 2 dpi, one at 4 dpi, four at 8 dpi, and one last 2 to 5 days. Ear skin cyanosis was observed in two pigs from 7 to 10 dpi. Two pigs showed chemosis and reddened conjunctiva from 8 to 15 dpi. Thereafter, body temperatures returned to normal, and all animals recovered and stayed healthy and alive until the end of the experiment. No pigs infected with HLJB1 died during the experiment.
). Anorexia and depression were seen in one pig from 11 to 13 dpi. All seven pigs exhibited respiratory symptoms to varying degrees; wheezing was observed in one pig at 2 dpi, one at 4 dpi, four at 8 dpi, and one last 2 to 5 days. Ear skin cyanosis was observed in two pigs from 7 to 10 dpi. Two pigs showed chemosis and reddened conjunctiva from 8 to 15 dpi. Thereafter, body temperatures returned to normal, and all animals recovered and stayed healthy and alive until the end of the experiment. No pigs infected with HLJB1 died during the experiment.
 |
Fig. (1) Body temperature of pigs following infection with HLJB1. Temperature ≥40°C was considered as fever. |
Serology of PRRSV
Anti-PRRSV IgG antibodies were detected in infected pig as early as 7 dpi and all infected pigs were found to be seropositive by 10 dpi. Thereafter, all infected pigs remained seropositive for PRRSV (Fig. 2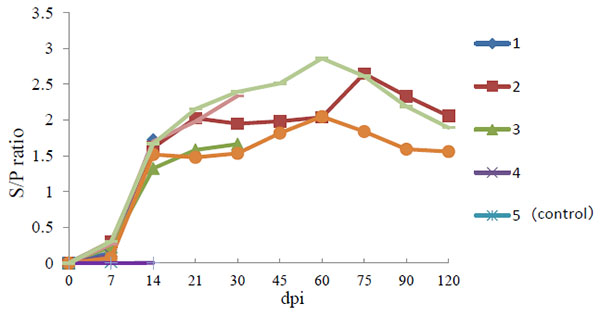 ). No anti-PRRSV IgG antibodies were detected in the serum of negative control pigs throughout the experiment.
). No anti-PRRSV IgG antibodies were detected in the serum of negative control pigs throughout the experiment.
 |
Fig. (2) Body temperature of pigs following infection with HLJB1. Temperature ≥40°C was considered as fever. |
RSV-Specific Serum IgG
Gross and histopathological tests
Gross pneumonic lesions were characterized by multifocal, tan-mottled areas with irregular and indistinct borders (Fig. 3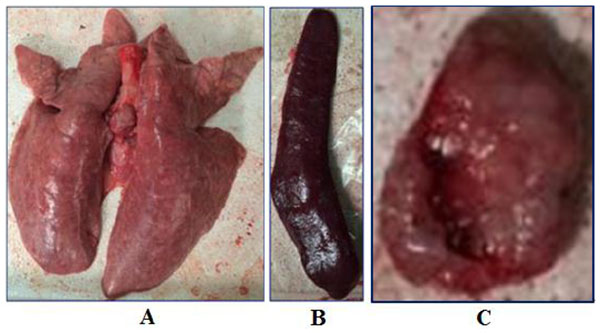 ). Lymph nodes were tan in color and enlarged. Spleens were enlarged and consolidated, with some containing infarcts. Characteristic microscopic lesions consisted of pulmonary consolidation and septal thickening with red blood cell infiltration, depletion of splenic lymphocytes, hyperplasia and macrophage activation, lymphocytic degeneration, necrosis, and red blood cell infiltration. Lymph nodes lesions included depletion of lymphocytes, and the cortex was infiltrated with a large number of red blood cells. Tonsils had necrotic foci (Fig. 4
). Lymph nodes were tan in color and enlarged. Spleens were enlarged and consolidated, with some containing infarcts. Characteristic microscopic lesions consisted of pulmonary consolidation and septal thickening with red blood cell infiltration, depletion of splenic lymphocytes, hyperplasia and macrophage activation, lymphocytic degeneration, necrosis, and red blood cell infiltration. Lymph nodes lesions included depletion of lymphocytes, and the cortex was infiltrated with a large number of red blood cells. Tonsils had necrotic foci (Fig. 4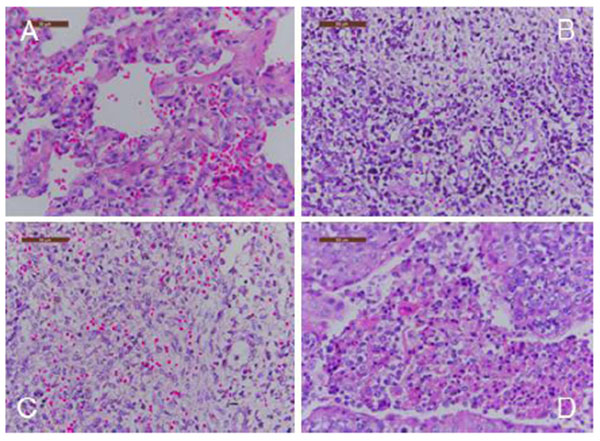 ).
).
 |
Fig. (3) Body temperature of pigs following infection with HLJB1. Temperature ≥40°C was considered as fever. |
 |
Fig. (4) Body temperature of pigs following infection with HLJB1. Temperature ≥40°C was considered as fever. |
Viremia
As there were a limited number of serum samples, the exact time viral titers were first detected in sera was not determined. However, the viral load in sera of HLJB1-infected pigs peaked at 7 dpi, and viremia in all pigs but one lasted until 21 dpi or longer (Fig. 5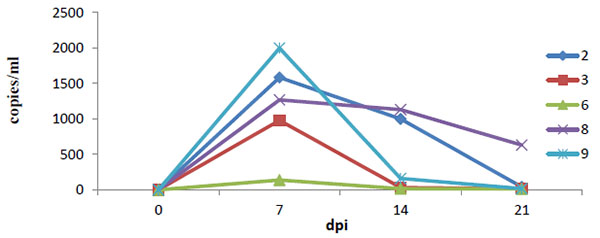 ).
).
 |
Fig. (5) Viral load in the serum as determined by quantitative real-time PCR. |
DISCUSSION
Lelystad virus is the prototype strain of type I PRRSV and represents viruses predominately originating in Europe. Experimental infection of Lelystad virus in pigs showed that the virus could induce mild transient pyrexia, dyspnea, tachypnea, and multifocal tan-mottled consolidation. Characteristic microscopic lung lesions consisted of type 2 pneumocyte hypertrophy and hyperplasia, necrotic debris and increased mixed inflammatory cells in alveolar spaces, alveolar septal infiltration with mononuclear cells, lymphadenopathy with follicular hypertrophy, hyperplasia, and necrosis [12Halbur PG, Paul PS, Frey ML, et al. Comparison of the pathogenicity of two USporcine reproductive and respiratory syndrome virus isolates with thatof the Lelystad virus. Vet Pathol 1995; 32: 648-60.
[http://dx.doi.org/10.1177/030098589503200606] ]. In contrast, the newly emerged East European subtype 3 PRRSV strain Lena showed pathogenic severity in conventional pigs. High fever, anorexia, and depression were prominent signs in most pigs inoculated with Lena between 2 and 28 dpi. Fever (temperature range, 40–42.1°C and lasting for several weeks), prolonged viremia (lasting until 28 dpi), co-infection with bacterial pathogens, abnormally high viral load in sera (ranging from 104.0 to 106.1 TCID50/ml at 10 dpi and 104.3 to 106.0 TCID50/ml at 14 dpi.), high viral titers in tissues, and high mortality were prominent findings [17Karniychuk UU, Geldhof M, Vanhee M, Van Doorsselaere J, Saveleva TA, Nauwynck HJ. Pathogenesis and antigenic characterization of a new EastEuropean subtype 3 porcine reproductive and respiratory syndromevirus isolate. BMC Vet Res 2010; 6: 30.
[http://dx.doi.org/10.1186/1746-6148-6-30] ]. Additionally, Lyoo et al. recently described two field cases of type 1 PRRSV infection with unusually severe pathogenicity that occurred in South Korea [15Lyoo KS, Yeom M, Choi JY, Park JH, Yoon SW, Song D. Unusual severe cases of type 1 porcine reproductive and respiratory syndrome virus (PRRSV) infection in conventionally reared pigs in South Korea. BMC Vet Res 2015; 11: 272.
[http://dx.doi.org/10.1186/s12917-015-0584-5] ]. Severe respiratory distress was seen in pigs of the grower-to-finish unit, with mortality rates ranging from 22% and 50% [15Lyoo KS, Yeom M, Choi JY, Park JH, Yoon SW, Song D. Unusual severe cases of type 1 porcine reproductive and respiratory syndrome virus (PRRSV) infection in conventionally reared pigs in South Korea. BMC Vet Res 2015; 11: 272.
[http://dx.doi.org/10.1186/s12917-015-0584-5] ].
In the present study, the pathogenicity of a newly emerging type I PRRSV strain (HLJB1) in mainland China was evaluated in young pigs. Like other classic type 1 PRRSV isolates, the novel Chinese type 1 strain HLJB1 induced mild clinical signs such as pyrexia and dyspnea upon experimental infection of young pigs. Gross pneumonic lesions with enlarged lymph nodes and spleen were observed. Characteristic microscopic lesions consisted of pulmonary consolidation, depletion of splenic lymphocytes, hyperplasia and macrophage activation, lymphocytic degeneration, and necrosis. The clinical signs and pathological changes were all similar to that of type 1 PRRSV strains in previous reports. Nevertheless, HLJB1 caused lower viremia compared with other classic type 1 isolates, and viremia in most pigs infected with HLJB1 lasted only 2–3 weeks. As higher and prolonged viremia is one of the prominent characteristics of highly pathogenic type I PRRSV strains, the results in this study suggested that HLJB1 is less virulent than other type I strains. Despite lower viremia, HLJB1 induced a good immune response in infected pigs (Fig. 2), suggesting the potential of type I PRRSV HLJB1 as a vaccine candidate.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest related to the present study.
ACKNOWLEDGEMENTS
The authors thank Dr. Xiuling Yu, Pulike Biological Engineering, Inc., for technical assistance with histopathological tests.
REFERENCES
| [1] | Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch Virol 1997; 142: 629-33. |
| [2] | Meulenberg JJ, Hulst MM, de Meijer EJ, et al. Lelystad virus, the causative agent of porcine epidemicabortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology 1993; 192(1): 62-72. [http://dx.doi.org/10.1006/viro.1993.1008] |
| [3] | Meng XJ, Paul PS, Halbur PG, Lum MA. Phylogenetic analyses of the putative M (ORF-6)-gene and N (ORF-7)-gene of porcine reproductive and respiratory syndrome virus (PRRSV): implication for the existence of 2genotypes of PRRSV in the USA and Europe. Arch Virol 1995; 140: 745-55. [http://dx.doi.org/10.1007/BF01309962] |
| [4] | Nelsen CJ, Murtaugh MP, Faaberg KS. Porcine reproductive and respiratory syndrome virus comparison: divergent evolution on two continents. J Virol 1999; 73: 270-80. |
| [5] | Wensvoort G, de Kluyver EP, Luijtze EA, et al. Antigenic comparison of Lelystad virus and swine infertility and respiratory syndrome (SIRS) virus. J Vet Diagn Invest 1992; 4: 134-8. [http://dx.doi.org/10.1177/104063879200400203] |
| [6] | Wieczorek-Krohmer M, Weiland F, Conzelmann K, et al. Porcine reproductive and respiratory syndrome virus (PRRSV): monoclonal antibodies detect common epitopes on two viral proteins of European and U.S. isolates. Vet Microbiol 1996; 51: 257-66. [http://dx.doi.org/10.1016/0378-1135(96)00047-8] |
| [7] | Andreyev VG, Wesley RD, Mengeling WL, Vorwald AC, Lager KM. Genetic variation and phylogenetic relationships of 22 porcine reproductive and respiratory syndrome virus (PRRSV) field strains based on sequence analysis of open reading frame 5. Arch Virol 1997; 142(5): 993-1001. [http://dx.doi.org/10.1007/s007050050134] |
| [8] | Murtaugh MP, Stadejek T, Abrahante JE, Lam TT, Leung FC. The ever-expanding diversity of porcine reproductive and respiratory syndrome virus. Virus Res 2010; 154(1-2): 18-30. [http://dx.doi.org/10.1016/j.virusres.2010.08.015] |
| [9] | Stadejek T, Oleksiewicz MB, Scherbakov AV, et al. Definition of subtypes in the European genotype of porcine reproductive and respiratory syndrome virus: nucleocapsid characteristics and geographical distribution in Europe. Arch Virol 2008; 153: 1479-88. [http://dx.doi.org/10.1007/s00705-008-0146-2] |
| [10] | Amonsin A, Kedkovid R, Puranaveja S, Wongyanin P, Suradhat S, Thanawongnuwech R. Comparative analysis of complete nucleotide sequence of porcine reproductive and respiratory syndrome virus (PRRSV) isolates in Thailand (US and EU genotypes). Virol J 2009; 6: 143. [http://dx.doi.org/10.1186/1743-422X-6-143] |
| [11] | Balka G. Hornya k, A., Ba lint, A., Kiss, I., Kecskeme ti, S., Bakonyi, T. Rusvai, M. Genetic diversity of porcine reproductive and respiratory syndrome virus strains circulating in Hungarian swine herds. Vet Microbiol 2008; 127: 128-35. [http://dx.doi.org/10.1016/j.vetmic.2007.08.001] |
| [12] | Halbur PG, Paul PS, Frey ML, et al. Comparison of the pathogenicity of two USporcine reproductive and respiratory syndrome virus isolates with thatof the Lelystad virus. Vet Pathol 1995; 32: 648-60. [http://dx.doi.org/10.1177/030098589503200606] |
| [13] | Martínez-Lobo FJ, Díez-Fuertes F, Segalés J, et al. Comparative pathogenicity of type 1 and type 2 isolates ofporcine reproductive and respiratory syndrome virus (PRRSV) in a youngpig infection model. Vet Microbiol 2011; 154: 58-68. [http://dx.doi.org/10.1016/j.vetmic.2011.06.025] |
| [14] | Van der Linden IF, Voermans JJ, van der Linde-Bril EM, Bianchi AT, Steverink PJ. Virological kinetics and immunological responses to a porcine reproductive and respiratory syndrome virus infection of pigs at different ages. Vaccine 2003; 21: 1952-7. [http://dx.doi.org/10.1016/S0264-410X(02)00822-8] |
| [15] | Lyoo KS, Yeom M, Choi JY, Park JH, Yoon SW, Song D. Unusual severe cases of type 1 porcine reproductive and respiratory syndrome virus (PRRSV) infection in conventionally reared pigs in South Korea. BMC Vet Res 2015; 11: 272. [http://dx.doi.org/10.1186/s12917-015-0584-5] |
| [16] | Tian K, Yu X, Zhao T, et al. Emergence of fatal PRRSV variants: unparalleled outbreaks ofatypical PRRS in China and molecular dissection of the unique hallmark. PLoS One 2007; 2: e526. [http://dx.doi.org/10.1371/journal.pone.0000526] |
| [17] | Karniychuk UU, Geldhof M, Vanhee M, Van Doorsselaere J, Saveleva TA, Nauwynck HJ. Pathogenesis and antigenic characterization of a new EastEuropean subtype 3 porcine reproductive and respiratory syndromevirus isolate. BMC Vet Res 2010; 6: 30. [http://dx.doi.org/10.1186/1746-6148-6-30] |
| [18] | Guo BQ, Chen ZS, Liu WX, Cui YZ. Isolation of PRRSV from aborted fetus with typical clinical PRRS manifestations. Chin J Prev Vet Med 1996; 2: 1-5. [in Chinese]. |
| [19] | Gao ZQ, Guo X, Yang HC. Genomic characterization of two Chinese isolates of porcine respiratory and reproductive syndrome virus. Arch Virol 2004; 149: 1341-51. [http://dx.doi.org/10.1007/s00705-004-0292-0] |
| [20] | Li Y, Wang X, Jiang P, et al. Genetic variation analysis of porcine reproductive and respiratory syndrome virus isolated in China from 2002 to 2007 based on ORF5. Vet Microbiol 2009; 138: 150-5. b [http://dx.doi.org/10.1016/j.vetmic.2009.03.001] |
| [21] | Zhou L, Chen S, Zhang J, et al. Molecular variation analysis of porcine reproductive and respiratory syndrome virus in China. Virus Res 2009; 145: 97-105. a [http://dx.doi.org/10.1016/j.virusres.2009.06.014] |
| [22] | Wasilk A, Callahan JD, Christopher-Hennings J, et al. Detection of U.S., Lelystad, and European-like porcine reproductive and respiratory syndrome viruses and relative quantitation in boar semen and serum samples by real-time PCR. J Clin Microbiol 2004; 42: 4453-61. [http://dx.doi.org/10.1128/JCM.42.10.4453-4461.2004] |



