- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Virology Journal
(Discontinued)
ISSN: 1874-3579 ― Volume 15, 2021
The Evolutionary Significance of Generalist Viruses with Special Emphasis on Plant Viruses and their Hosts
Mayank Kumar1, Ruchika Bharti2, Tushar Ranjan3, *
Abstract
The host range of a virus is defined as the number of species a virus potentially infects. The specialist virus infects one or few related species while the generalist virus infects several different species, possibly in different families. Origin of generalist viruses from their specialist nature and the expansion of the host range of the generalist virus occur with the host shift event in which the virus encounters and adapts to a new host. Host shift events have resulted in the majority of the newly emerging viral diseases. This review discusses the advantages and disadvantages of generalist over specialist viruses and the unique features of plant viruses and their hosts that result in a higher incidence of generalist viruses in plants.
Article Information
Identifiers and Pagination:
Year: 2020Volume: 14
First Page: 22
Last Page: 29
Publisher Id: TOVJ-14-22
DOI: 10.2174/1874357902014010022
Article History:
Received Date: 20/06/2020Revision Received Date: 13/09/2020
Acceptance Date: 19/10/2020
Electronic publication date: 31/12/2020
Collection year: 2020
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at Department of Molecular Biology and Genetic Engineering, Bihar Agricultural University, Sabour, Bhagalpur 813 210, India; E-mail: mail2tusharranjan@gmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 20-06-2020 |
Original Manuscript | The Evolutionary Significance of Generalist Viruses with Special Emphasis on Plant Viruses and their Hosts | |
1. INTRODUCTION
Viruses can infect all the life forms from archaebacteria to eubacteria, from Protista to algae, from plants to animals [1Payne S. Viruses: From Understanding to Investigation 2018.]. They cause different diseases in plants and animals, accounting for huge economic loss in addition to the loss of lives [2Roossinck MJ. Lifestyles of plant viruses. Philos Trans R Soc Lond B Biol Sci 2010; 365(1548): 1899-905.
[http://dx.doi.org/10.1098/rstb.2010.0057] [PMID: 20478885] , 3Strauss E, Strauss J. Viruses and Human Disease 2nd ed. 2008.]. Like any other pathogens, viruses need transmission routes for their spread. Animal viruses use different transmission routes such as micro-droplets through the air, food, water, direct physical contact and vectors [4Payne S. Virus Transmission and Epidemiology.Viruses: From Understanding to Investigation 2018; 53-60.]. Since plants are sessile, most plant viruses use vectors for their transmission from one plant to another [5Whitfield AE, Falk BW, Rotenberg D. Insect vector-mediated transmission of plant viruses. Virology 2015; 479-480: 278-89.
[http://dx.doi.org/10.1016/j.virol.2015.03.026] [PMID: 25824478] ]. Other modes of transmission include direct entry through the site of injury caused by agricultural practices and environmental factors, vegetative propagation from infected parts, and infected seeds [6Stevens WA. Transmission of Plant Viruses.Virology of Flowering Plants 1983; 41-68.
[http://dx.doi.org/10.1007/978-1-4757-1251-3_3] ]. The most common vectors of plant viruses are insects, in addition to nematodes, arthropods, and fungi. These vectors acquire viruses from infected plants and release them when they feed on healthy plants.
A virus can infect one or more hosts and this is determined by the host range of the virus. The host range indicates the number of different hosts a virus can infect. Some viruses are highly specific and infect one or related hosts (specialist virus), while others infect more than one unrelated host (generalist virus). Generalist viruses can have a narrow host range when they infect few hosts or a wide host range when they infect a large number of hosts [7Elena SF, Agudelo-Romero P, Lalić J. The evolution of viruses in multi-host fitness landscapes. Open Virol J 2009; 3: 1-6.
[http://dx.doi.org/10.2174/1874357900903010001] [PMID: 19572052] ]. Examples of specialist viruses are dengue and mumps viruses, which infect only humans, while examples of generalist viruses are cucumber mosaic virus, which infects several species of plants, influenza A virus, which infects birds and several mammal species, and canine distemper virus, which infects several species of mammals [7Elena SF, Agudelo-Romero P, Lalić J. The evolution of viruses in multi-host fitness landscapes. Open Virol J 2009; 3: 1-6.
[http://dx.doi.org/10.2174/1874357900903010001] [PMID: 19572052] , 8Parrish CR, Holmes EC, Morens DM, et al. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol Mol Biol Rev 2008; 72(3): 457-70.
[http://dx.doi.org/10.1128/MMBR.00004-08] [PMID: 18772285] ].
The host shift event is defined as an event where a pathogen encounters and adapts to a new host. The host shift is a significant event in virology. It allows the specialist virus to become a generalist by expanding its host range. The host shift event can result in the emergence of new diseases in both plants and animals and may cause huge economic loss and loss of lives. Pathogens host shifts have resulted in most of the recent emerging diseases in animals and plants [8Parrish CR, Holmes EC, Morens DM, et al. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol Mol Biol Rev 2008; 72(3): 457-70.
[http://dx.doi.org/10.1128/MMBR.00004-08] [PMID: 18772285] , 10Morens DM, Fauci AS. Emerging infectious diseases: Threats to human health and global stability. PLoS Pathog 2013; 9(7)e1003467
[http://dx.doi.org/10.1371/journal.ppat.1003467] [PMID: 23853589] ]. For example, human diseases like Avian flu, AIDS, SAARS, Dengue originated because of host shift events [8Parrish CR, Holmes EC, Morens DM, et al. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol Mol Biol Rev 2008; 72(3): 457-70.
[http://dx.doi.org/10.1128/MMBR.00004-08] [PMID: 18772285] , 10Morens DM, Fauci AS. Emerging infectious diseases: Threats to human health and global stability. PLoS Pathog 2013; 9(7)e1003467
[http://dx.doi.org/10.1371/journal.ppat.1003467] [PMID: 23853589] ]. In plants, Pepino mosaic virus, Tomato leaf curl New Delhi virus, Begomoviruses and Tospoviruses are rapidly expanding their host range, posing a serious threat to agricultural production [9Maria R, Rojas RLG. Emerging plant viruses: A diversity of mechanisms and opportunities.Plant Virus Evolution 2008; 27-51.
-13Moriones E, Praveen S, Chakraborty S. Tomato leaf curl new delhi virus: An emerging virus complex threatening vegetable and fiber crops. Viruses 2017; 9(10)E264
[http://dx.doi.org/10.3390/v9100264] [PMID: 28934148] ].
Zoonotic diseases (zoonoses) are transmitted naturally from animals, mostly vertebrates, to humans. Zoonoses may be bacterial, viral, fungal, protozoal or any other pathogen. A study published by Taylor et al. [14Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci 2001; 356(1411): 983-9.
[http://dx.doi.org/10.1098/rstb.2001.0888] [PMID: 11516376] ] found that 61% of the pathogens that infect humans are zoonotic and 175 are associated with the emerging diseases. Among the pathogens associated with emerging diseases, 75% are zoonotic. The analysis revealed that zoonotic pathogens are twice as likely to cause emerging diseases as non-zoonotic pathogens. These pathogens are able to break the barriers of host shift events. Another study by Jones et al. [15Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature 2008; 451(7181): 990-3.
[http://dx.doi.org/10.1038/nature06536] [PMID: 18288193] ] found a similar result. They also found that majority of the zoonoses linked emerging diseases (71.8%) originate in wildlife and their numbers are increasing over time. However, certain species, such as bats, serve as an exception and act as a reservoir for zoonoses [16Luis AD, Hayman DT, O'Shea TJ, et al. 2013; A comparison of bats and rodents as reservoirs of zoonotic viruses: Are bats special? Proceedings Biological sciences 280(1756): 2012-753.
[http://dx.doi.org/10.1098/rspb.2012.2753] ]. Examples of some important zoonotic viral pathogens include Rabies virus, HIV, avian and swine influenza virus, SARS virus, MERS virus, Ebola virus, Nipah virus, Chikugunya virus [17Bengis RG, Leighton FA, Fischer JR, Artois M, Mörner T, Tate CM. The role of wildlife in emerging and re-emerging zoonoses. Rev Sci Tech 2004; 23(2): 497-511.
[PMID: 15702716] -19Wang LF, Crameri G. Emerging zoonotic viral diseases. Rev Sci Tech 2014; 33(2): 569-81.
[http://dx.doi.org/10.20506/rst.33.2.2311] [PMID: 25707184] ], and the most recent COVID-19 virus [20Perlman S. Another decade, another coronavirus. N Engl J Med 2020; 382(8): 760-2.
[http://dx.doi.org/10.1056/NEJMe2001126] [PMID: 31978944] , 21Salata C, Calistri A, Parolin C, Palù G. 2019; Coronaviruses: A paradigm of new emerging zoonotic diseases. Pathogens and disease 77(9): ftaa006.
[http://dx.doi.org/10.1093/femspd/ftaa006] ].
Orthoreovirus is an example zoonotic virus having a wide host range. It mainly infects mammals and some non-mammalian species of reptiles and birds [22Chua KB, Voon K, Yu M, Keniscope C, Abdul Rasid K, Wang LF. Investigation of a potential zoonotic transmission of orthoreovirus associated with acute influenza-like illness in an adult patient. PLoS One 2011; 6(10)e25434
[http://dx.doi.org/10.1371/journal.pone.0025434] [PMID: 22022394] ]. They belong to reovirus and contain segmented ds RNA genome and can lead to the emergence of a pandemic strain via mutation and/or reassortment of the genome. Recently, members of orthoreovirus have caused zoonotic infection in humans [23Chua KB, Crameri G, Hyatt A, et al. A previously unknown reovirus of bat origin is associated with an acute respiratory disease in humans. Proc Natl Acad Sci USA 2007; 104(27): 11424-9.
[http://dx.doi.org/10.1073/pnas.0701372104] [PMID: 17592121] , 24Chua KB, Voon K, Crameri G, et al. Identification and characterization of a new orthoreovirus from patients with acute respiratory infections. PLoS One 2008; 3(11)e3803
[http://dx.doi.org/10.1371/journal.pone.0003803] [PMID: 19030226] ]. Another interesting example of a virus that causes recurrent zoonotic transmission is the influenza virus. Avian and swine influenza virus subtype A have caused sporadic infections and pandemics in the past. Aquatic birds and poultry are primary and the natural reservoirs of the Avian Influenza A virus (Bird flu). Strains that have caused the pandemics are avian influenza virus subtypes A(H5N1), A(H7N9), and A(H9N2) and swine influenza virus subtypes A(H1N1), A(H1N2) and A(H3N2). The entry of the influenza virus inside the cell is mediated by the interaction of the viral glycoprotein, hemagglutinin (HA), and the host cell surface sialic acid receptor. Since sialic acid receptors are found on the surface of human respiratory tract cells, zoonotic transmission becomes possible [25Dou D, Revol R, Östbye H, Wang H, Daniels R. Influenza a virus cell entry, replication, virion assembly and movement. Front Immunol 2018; 9: 1581.
[http://dx.doi.org/10.3389/fimmu.2018.01581] [PMID: 30079062] , 26Richard M, de Graaf M, Herfst S. Avian influenza a viruses: From zoonosis to pandemic. Future Virol 2014; 9(5): 513-24.
[http://dx.doi.org/10.2217/fvl.14.30] [PMID: 25214882] ].
On the other hand of the host range of viruses, lie specialist viruses. Hepatitis Delta virus (HDV) is an interesting example of it. It primarily infects humans and is one of the five hepatitis viruses. HDV has some unique features among animal viruses. It is the smallest animal virus known and contains a single-stranded circular RNA genome of just 1.7 kb. It shows similarity to viroids in genome architecture, mode of genome replication and interaction of the genome with the host proteins. HDV is a defective virus since it requires co-infection with the Hepatitis B virus for its life-cycle. Thus, HDV infection in humans occurs only in the presence of HBV infection (Superinfection) [27Watashi K, Wakita T. Hepatitis b virus and hepatitis d virus entry, species specificity, and tissue tropism. Cold Spring Harb Perspect Med 2015; 5(8)a021378
[http://dx.doi.org/10.1101/cshperspect.a021378] [PMID: 26238794] ].
Hence, works towards understanding the host-shift events of viruses have gained momentum in recent years. This review will summarise the evolutionary advantages and disadvantages of generalist viruses over specialist viruses, adaptive challenges faced by a virus during a host shift event and the unique features of plants and their viruses that promote the generalist nature of plant viruses.
2. ADVANTAGES AND DISADVANTAGES OF GENERALIST VIRUSES
Both generalist and specialist viruses are found in nature, and it is useful to compare the evolutionary advantages and disadvantages of generalist viruses over specialist viruses, which can be summarized as follows:
2.1. Generalist Viruses have Higher Chances of Propagation
Any living organism aims to increase its population and this also applies to viruses. After completing the life cycle in a host, the virus exits and infects the same or a different host to continue the life cycle. Generalist viruses have a higher probability of finding the correct host for infection since they infect more number of species than the specialist viruses [8Parrish CR, Holmes EC, Morens DM, et al. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol Mol Biol Rev 2008; 72(3): 457-70.
[http://dx.doi.org/10.1128/MMBR.00004-08] [PMID: 18772285] ]. In addition, if a virus has multiple hosts, one of the hosts can act as a reservoir where, in the absence of a preferred host, the virus stays in an alternate host until the preferred host becomes available. This increases the chances of successful propagation of a generalist virus. For example, Potato leafroll virus and Potato virus Y make use of a weed, hairy nightshade (Solanum sarrachoides), as a viral reservoir and this increases virus infection of potato plants [28Srinivasan R, Alvarez JM, Bosque-Pérez NA, Eigenbrode SD, Novy RG. Effect of an alternate weed host, hairy nightshade, Solanum sarrachoides, on the biology of the two most important potato leafroll virus (Luteoviridae: Polerovirus) vectors, Myzus persicae and Macrosiphum euphorbiae (Aphididae: Homoptera). Environ Entomol 2008; 37(2): 592-600.
[PMID: 18419933] , 29Cervantes FA, Alvarez JM. Within plant distribution of Potato Virus Y in hairy nightshade (Solanum sarrachoides): An inoculum source affecting PVY aphid transmission. Virus Res 2011; 159(2): 194-200.
[http://dx.doi.org/10.1016/j.virusres.2011.05.003] [PMID: 21601597] ]. Similarly, bats serve as a reservoir for several important human viruses like Ebola and Nipah viruses [30Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Bats: Important reservoir hosts of emerging viruses. Clin Microbiol Rev 2006; 19(3): 531-45.
[http://dx.doi.org/10.1128/CMR.00017-06] [PMID: 16847084] ].
2.2. Generalist Viruses have Lower Extinction Threats
Viruses act as pathogens and hence, according to the Red Queen hypothesis, there is a race between a virus and its host, where on the one hand, the host aims to develop defense mechanisms that can either eliminate or render the virus harmless or less harmful [31Dawkins R, Krebs JR. Arms races between and within species. Proc R Soc Lond B Biol Sci 1979; 205(1161): 489-511.
[http://dx.doi.org/10.1098/rspb.1979.0081] [PMID: 42057] -34Rosenzweig ML, Brown JS, Vincent TL. Red Queens and ESS: The coevolution of evolutionary rates. Evol Ecol 1987; 1: 59-94.
[http://dx.doi.org/10.1007/BF02067269] ], but on the other hand, the virus aims to maximize its fitness in the host [35Anderson RM, May RM. Coevolution of hosts and parasites. Parasitology 1982; 85(Pt 2): 411-26.
[http://dx.doi.org/10.1017/S0031182000055360] [PMID: 6755367] ]. Fitness is a cumulative ability of the virus to utilize the host resources for its propagation, ability to counteract the host defense network by increasing its virulence and transmission potential. Hence, with time the host may evolve to become resistant to a virus. This has been observed in plants [32de Ronde D, Butterbach P, Kormelink R. Dominant resistance against plant viruses. Front Plant Sci 2014; 5: 307.
[http://dx.doi.org/10.3389/fpls.2014.00307] [PMID: 25018765] ]. For example, several plant species developed resistance to specific viruses through resistance genes that are either dominant or recessive in nature. Dominant resistance genes act by synthesising protein products that bind to the avirulence (Avr) proteins of the viruses, rendering the virus inactive. Recessive resistance genes code for the mutated forms of the host factors indispensable for viral propagation, as a result, the virus fails to utilise them. If both the copies of a recessive resistance gene are mutated, the virus fails to propagate in the host. Therefore, if a host develops resistance then it will pose a serious extinction threat to a specialist virus, whereas the generalist virus can complete its life cycle in its alternate host(s). In addition, if the population of one of the hosts dwindles or if the host gets extinct, a generalist virus can complete its life cycle in other hosts. However, a specialist virus may face an extinction risk with the extinction of its host. Thus, having more than one host may increase the chances of viral survival.
2.3. Generalist Nature of Viruses Aids in their Evolution
Generalist viruses propagate in more than one host. Different hosts pose varying selection pressure on the virus. This leads the virus to adapt differently in different hosts and thus, the selection of unique genotypes of the virus in each host. This can result in the evolution of a new species of the virus. The evolution aims to improve the fitness of the virus in the given host [36Froissart R, Roze D, Uzest M, Galibert L, Blanc S, Michalakis Y. Recombination every day: Abundant recombination in a virus during a single multi-cellular host infection. PLoS Biol 2005; 3(3)e89
[http://dx.doi.org/10.1371/journal.pbio.0030089] [PMID: 15737066] ]. Nonetheless, the evolved species of the virus may show fitness trade-offs in the original hosts [7Elena SF, Agudelo-Romero P, Lalić J. The evolution of viruses in multi-host fitness landscapes. Open Virol J 2009; 3: 1-6.
[http://dx.doi.org/10.2174/1874357900903010001] [PMID: 19572052] , 37Longdon B, Brockhurst MA, Russell CA, Welch JJ, Jiggins FM. The evolution and genetics of virus host shifts. PLoS Pathog 2014; 10(11)e1004395
[http://dx.doi.org/10.1371/journal.ppat.1004395] [PMID: 25375777] ].
2.4. Opportunity for the Generalist Virus to Acquire New Genetic Material
The infection of a host with more than one virus has been observed [38Syverton JT, Berry GP. Multiple virus infection of single host cells. J Exp Med 1947; 86(2): 145-52.
[http://dx.doi.org/10.1084/jem.86.2.145] [PMID: 19871662] ]. Hence, a generalist virus may be exposed to different sets of genetic material in different hosts, given the host is infected with more than one type of virus at one time. However, a specialist virus will have limited exposure to foreign DNA. This provides the generalist virus higher ability to bring variations through recombination and reassortment and may lead to an evolution of a new strain/species of the virus. However, there is limited literature on this aspect of virus evolution. Reassortment was observed in the evolution of the Influenza virus since it has a segmented genome and hence viruses with different genotypes can exchange nucleic acid segments [39De Clercq E. Antiviral agents active against influenza a viruses. Nat Rev Drug Discov 2006; 5(12): 1015-25.
[http://dx.doi.org/10.1038/nrd2175] [PMID: 17139286] -41Reid AH, Taubenberger JK. The origin of the 1918 pandemic influenza virus: A continuing enigma. J Gen Virol 2003; 84(Pt 9): 2285-92.
[http://dx.doi.org/10.1099/vir.0.19302-0] [PMID: 12917448] ].
2.5. Generalist Viruses have Higher Fitness in a Dynamic Environment
In a dynamic environment, where fluctuation in biotic and abiotic factors occurs, generalist viruses have higher chances of survival. This is because generalist viruses thrive on more than one host and different hosts pose varying selection pressure on the virus. This allows the virus to adapt differently in different hosts leading to higher standing variation at any point in time. This helps the generalist virus adapts faster in a dynamic environment [42Woolhouse ME, Taylor LH, Haydon DT. Population biology of multihost pathogens. Science 2001; 292(5519): 1109-12.
[http://dx.doi.org/10.1126/science.1059026] [PMID: 11352066] , 43Whitlock MC. The Red Queen Beats the Jack-Of-All-Trades: The Limitations on the Evolution of Phenotypic Plasticity and Niche Breadth. Am Nat 1996; 148: 565-77.
[http://dx.doi.org/10.1086/285902] ].
3. DISADVANTAGES OF GENERALIST VIRUSES OVER SPECIALIST VIRUSES
3.1. Generalist Viruses Suffer from Fitness Trade-offs
Co-evolution of the virus and its host results in the specialisation of the virus, which helps the virus to improve its fitness in the host. As a result, a specialist virus can evolve to achieve maximum fitness in its host [42Woolhouse ME, Taylor LH, Haydon DT. Population biology of multihost pathogens. Science 2001; 292(5519): 1109-12.
[http://dx.doi.org/10.1126/science.1059026] [PMID: 11352066] , 43Whitlock MC. The Red Queen Beats the Jack-Of-All-Trades: The Limitations on the Evolution of Phenotypic Plasticity and Niche Breadth. Am Nat 1996; 148: 565-77.
[http://dx.doi.org/10.1086/285902] ]. However, it will be a daunting task for a generalist virus to maximise its fitness in each of its hosts [44Bera S, Fraile A, García-Arenal F. Analysis of fitness trade-offs in the host range expansion of an rna virus, tobacco mild green mosaic virus. J Virol 2018; 92(24): 92.
[http://dx.doi.org/10.1128/JVI.01268-18] [PMID: 30257999] ] since higher fitness in a host may cause fitness trade-offs in other hosts and vice-versa [7Elena SF, Agudelo-Romero P, Lalić J. The evolution of viruses in multi-host fitness landscapes. Open Virol J 2009; 3: 1-6.
[http://dx.doi.org/10.2174/1874357900903010001] [PMID: 19572052] ]. Therefore, a generalist virus evolves to acquire fitness, which allows it to propagate in each of the hosts successfully, albeit with different fitness. The lower fitness of the generalist virus may be disadvantageous when it has to compete with another virus having higher fitness in a given host. The fitness cost of its adaptation in a new host could even lead to the loss of the original host(s) [7Elena SF, Agudelo-Romero P, Lalić J. The evolution of viruses in multi-host fitness landscapes. Open Virol J 2009; 3: 1-6.
[http://dx.doi.org/10.2174/1874357900903010001] [PMID: 19572052] , 45Sharp PM, Hahn BH. The evolution of HIV-1 and the origin of AIDS. Philos Trans R Soc Lond B Biol Sci 2010; 365(1552): 2487-94.
[http://dx.doi.org/10.1098/rstb.2010.0031] [PMID: 20643738] ].
3.2. Generalist Viruses are Disadvantageous in a Stable Environment
Over time, evolution should help the virus increase its fitness in the host until it achieves an optimum possible fitness. Since the generalist virus propagates in more than one host, it has to face different selection pressures, thus may not be able to maximise its fitness, as explained earlier. On the other hand, the specialist virus will evolve to achieve maximum fitness in its host. Hence, a stable environment favours the evolution of specialist viruses [46Baronchelli A, Chater N, Christiansen MH, Pastor-Satorras R. Evolution in a changing environment. PLoS One 2013; 8(1)e52742
[http://dx.doi.org/10.1371/journal.pone.0052742] [PMID: 23326355] ].
4. BARRIERS OF A HOST SHIFT EVENT BY A VIRUS
The evolution of the generalist virus from its specialist nature and expansion of the host range of the generalist virus occurs due to a host shift event where the virus encounters and adapts to a new host. However, there are certain challenges for a successful host shift event by a lytic virus (Fig. 1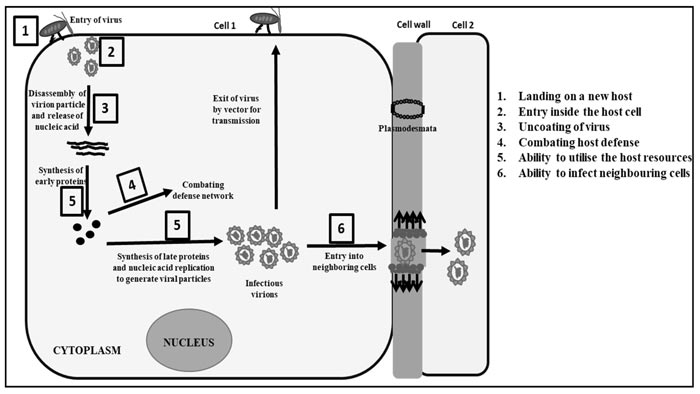 ) [37Longdon B, Brockhurst MA, Russell CA, Welch JJ, Jiggins FM. The evolution and genetics of virus host shifts. PLoS Pathog 2014; 10(11)e1004395
) [37Longdon B, Brockhurst MA, Russell CA, Welch JJ, Jiggins FM. The evolution and genetics of virus host shifts. PLoS Pathog 2014; 10(11)e1004395
[http://dx.doi.org/10.1371/journal.ppat.1004395] [PMID: 25375777] ]. These are as follows:
(1) The landing of a virus on a new host.
(2) Virus entry inside the cell of the new host.
(3) Uncoating of the virus inside the cell to release the genomic nucleic acid.
(4) Overcoming the host defense network.
(5) The ability of the virus to utilize the host resources for multiplication.
(6) The ability of the virus to be transmitted within the host.
5. UNIQUE CASE OF PLANTS AND THEIR VIRUSES
During a host shift event, a virus has to overcome certain challenges (Fig. 1 ). Yet, host shift events are common in nature and lead to the evolution of generalist viruses both in animals and plants [7Elena SF, Agudelo-Romero P, Lalić J. The evolution of viruses in multi-host fitness landscapes. Open Virol J 2009; 3: 1-6.
). Yet, host shift events are common in nature and lead to the evolution of generalist viruses both in animals and plants [7Elena SF, Agudelo-Romero P, Lalić J. The evolution of viruses in multi-host fitness landscapes. Open Virol J 2009; 3: 1-6.
[http://dx.doi.org/10.2174/1874357900903010001] [PMID: 19572052] , 37Longdon B, Brockhurst MA, Russell CA, Welch JJ, Jiggins FM. The evolution and genetics of virus host shifts. PLoS Pathog 2014; 10(11)e1004395
[http://dx.doi.org/10.1371/journal.ppat.1004395] [PMID: 25375777] , 47Power AG, Flecker AS. Virus Specificity in Disease Systems: Are Species Redundant?The Importance of Species: Perspectives on Expendability and Triage 2003; 330-47.
[http://dx.doi.org/10.1515/9781400866779-023] , 48Bandín I, Dopazo CP. Host range, host specificity and hypothesized host shift events among viruses of lower vertebrates. Vet Res (Faisalabad) 2011; 42(1): 67.
[http://dx.doi.org/10.1186/1297-9716-42-67] [PMID: 21592358] ]. Research has shown that generalist viruses are common among plant viruses [47Power AG, Flecker AS. Virus Specificity in Disease Systems: Are Species Redundant?The Importance of Species: Perspectives on Expendability and Triage 2003; 330-47.
[http://dx.doi.org/10.1515/9781400866779-023] ]. The following are proposed possible reasons that could explain the higher occurrence of generalist viruses in plants:
5.1. Ability of Plant Viruses to Land on a Large Number of Non-host Species
The first step for a successful host shift by a virus is to land on a new host [8Parrish CR, Holmes EC, Morens DM, et al. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol Mol Biol Rev 2008; 72(3): 457-70.
[http://dx.doi.org/10.1128/MMBR.00004-08] [PMID: 18772285] ]. Viruses after completing the life cycle must exit the host and land on a new host to initiate a host shift event. Different viruses exploit different transmission agents like air, food, water, physical contacts between the hosts, vectors, etc. to enhance their spread [4Payne S. Virus Transmission and Epidemiology.Viruses: From Understanding to Investigation 2018; 53-60.-6Stevens WA. Transmission of Plant Viruses.Virology of Flowering Plants 1983; 41-68.
[http://dx.doi.org/10.1007/978-1-4757-1251-3_3] ].
Plant viruses are transmitted mostly through vectors, the majority of which are insects, which are host generalists and feed on a wide variety of plants [7Elena SF, Agudelo-Romero P, Lalić J. The evolution of viruses in multi-host fitness landscapes. Open Virol J 2009; 3: 1-6.
[http://dx.doi.org/10.2174/1874357900903010001] [PMID: 19572052] ]. Other modes of transmission include the use of contaminated tools, vegetative propagation of infected plant parts, infected seed, etc [12Hanssen IM, Lapidot M, Thomma BP. Emerging viral diseases of tomato crops. Mol Plant Microbe Interact 2010; 23(5): 539-48.
[http://dx.doi.org/10.1094/MPMI-23-5-0539] [PMID: 20367462] ]. These routes allow plant viruses to land on a wide variety of non-host plants (Fig. 2a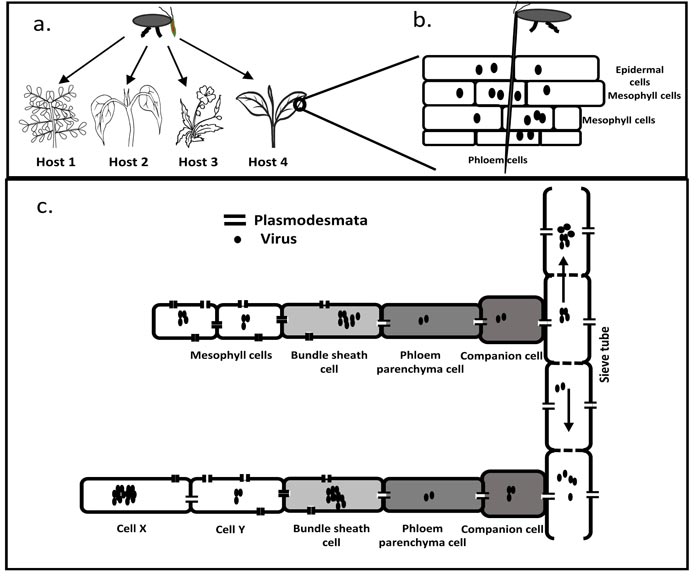 ). Similarly, animal viruses can also land on a wide variety of non-hosts as they are transmitted via micro-droplets through the air, contaminated food and water, direct physical contact and vectors [13Moriones E, Praveen S, Chakraborty S. Tomato leaf curl new delhi virus: An emerging virus complex threatening vegetable and fiber crops. Viruses 2017; 9(10)E264
). Similarly, animal viruses can also land on a wide variety of non-hosts as they are transmitted via micro-droplets through the air, contaminated food and water, direct physical contact and vectors [13Moriones E, Praveen S, Chakraborty S. Tomato leaf curl new delhi virus: An emerging virus complex threatening vegetable and fiber crops. Viruses 2017; 9(10)E264
[http://dx.doi.org/10.3390/v9100264] [PMID: 28934148] ].
5.2. Unrestricted Entry of Plant Viruses Inside the Plant Cells
After landing on a new host, the virus or its nucleic acid must enter inside the cell for carrying out the life cycle and thus successfully achieves a host shift, and this step is one of the most challenging steps in the successful host shift event. Animal viruses enter the cell by endocytosis or membrane fusion [49Payne S. Virus Interactions With the Cell.From Understanding to Investigation 2018; 23-35.], which requires specific molecular interactions between the viral surface proteins and host cell surface receptors. However, this specificity is a limitation for the entry of the animal viruses to non-host cells [8Parrish CR, Holmes EC, Morens DM, et al. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol Mol Biol Rev 2008; 72(3): 457-70.
[http://dx.doi.org/10.1128/MMBR.00004-08] [PMID: 18772285] ]. An analysis of 64 human viruses showed that those with the widest host range used protein receptors whose sequences were more conserved [50Woolhouse M, Scott F, Hudson Z, Howey R, Chase-Topping M. Human viruses: Discovery and emergence. Philos Trans R Soc Lond B Biol Sci 2012; 367(1604): 2864-71.
[http://dx.doi.org/10.1098/rstb.2011.0354] [PMID: 22966141] ]. Mutation in the attachment protein of phi-6 phage allowed them to become generalists by allowing them to bind to the receptors of the non-host cells and enter the cell [51Duffy S, Turner PE, Burch CL. Pleiotropic costs of niche expansion in the RNA bacteriophage phi 6. Genetics 2006; 172(2): 751-7.
[http://dx.doi.org/10.1534/genetics.105.051136] [PMID: 16299384] ]. In the case of canine parvovirus (CPV), two mutations in the Feline panleukopenia virus surface protein allowed it to bind to canine transferrin receptor and thus expanded its host range [52Hueffer K, Govindasamy L, Agbandje-McKenna M, Parrish CR. Combinations of two capsid regions controlling canine host range determine canine transferrin receptor binding by canine and feline parvoviruses. J Virol 2003; 77(18): 10099-105.
[http://dx.doi.org/10.1128/JVI.77.18.10099-10105.2003] [PMID: 12941920] , 53Shimakage MI, Yutsudo M, Hakura A, Toyoshima K. Isolation and characterization of host range mutants of avian sarcoma virus. J Gen Virol 1983; 64(Pt 4): 921-6.
[http://dx.doi.org/10.1099/0022-1317-64-4-921] [PMID: 6300310] ].
However, in the case of plant viruses, the plant cell has a rigid cell wall, and therefore, such viruses can not enter the cell similar to animal viruses. Plant viruses can only enter the cell through the mechanical injuries inflicted by either the vectors feeding on the plants, which are majorly host generalist or agricultural practices which can cause such injuries [54Balique F, Lecoq H, Raoult D, Colson P. Can plant viruses cross the kingdom border and be pathogenic to humans? Viruses 2015; 7(4): 2074-98.
[http://dx.doi.org/10.3390/v7042074] [PMID: 25903834] ]. As a result, plant viruses can easily gain access to a wide variety of non-host plant species (Fig. 2b ).
).
5.3. Lack of a Dedicated and Robust Immune System in Plants
Host cells have developed defense mechanisms to eliminate the introduction of viruses [55Barber GN. Host defense, viruses and apoptosis. Cell Death Differ 2001; 8(2): 113-26.
[http://dx.doi.org/10.1038/sj.cdd.4400823] [PMID: 11313713] ]. There is a race between the virus and its host where the host aims to evolve defense mechanisms that can eliminate or render the virus harmless or less harmful. On the other hand, the virus aims to evolve to counteract the host defense mechanisms and optimise its virulence that can increase its fitness in the host [31Dawkins R, Krebs JR. Arms races between and within species. Proc R Soc Lond B Biol Sci 1979; 205(1161): 489-511.
[http://dx.doi.org/10.1098/rspb.1979.0081] [PMID: 42057] , 34Rosenzweig ML, Brown JS, Vincent TL. Red Queens and ESS: The coevolution of evolutionary rates. Evol Ecol 1987; 1: 59-94.
[http://dx.doi.org/10.1007/BF02067269] ].
Once inside the cell, the virus faces the host defense network. Higher animals, such as vertebrates, have a highly developed immune system. It consists of organs and cells dedicated to mediate the defense. The immune cells scan for incoming threats and can move from one part of the body to another through motility and circulatory system. The system contains both innate and adaptive immunity. Innate immunity is broadly non-specific in nature, whereas adaptive immunity is highly specific and generates memory, which reduces the response time on successive infection by the same pathogen [56Yatim KM, Lakkis FG. A brief journey through the immune system. Clin J Am Soc Nephrol 2015; 10(7): 1274-81.
[http://dx.doi.org/10.2215/CJN.10031014] [PMID: 25845377] , 57Iriti M, Faoro F. Review of innate and specific immunity in plants and animals. Mycopathologia 2007; 164(2): 57-64.
[http://dx.doi.org/10.1007/s11046-007-9026-7] [PMID: 17554637] ].
On the contrary, the plant immune system lacks dedicated immune cells and organs. Rather, their defense network is located in each cell of the plant. Also, plants lack adaptive immunity and they only contain innate immunity, which is mainly provided by pattern recognition receptor (PRR), R-genes, RNA interference and Jasmonic acid signalling pathway [57Iriti M, Faoro F. Review of innate and specific immunity in plants and animals. Mycopathologia 2007; 164(2): 57-64.
[http://dx.doi.org/10.1007/s11046-007-9026-7] [PMID: 17554637] -59Jones JD, Dangl JL. The plant immune system. Nature 2006; 444(7117): 323-9.
[http://dx.doi.org/10.1038/nature05286] [PMID: 17108957] ]. RNA interference in plants is highly efficient in combating viral infections [60Rosa C, Kuo YW, Wuriyanghan H, Falk BW. RNA interference mechanisms and applications in plant pathology. Annu Rev Phytopathol 2018; 56: 581-610.
[http://dx.doi.org/10.1146/annurev-phyto-080417-050044] [PMID: 29979927] ]. But, it employs a conserved pathway and viruses can develop resistance against the conserved proteins of the RNAi in plants [61Voinnet O. Induction and suppression of RNA silencing: Insights from viral infections. Nat Rev Genet 2005; 6(3): 206-20.
[http://dx.doi.org/10.1038/nrg1555] [PMID: 15703763] ]. Resistance in one species may be able to provide resistance in related species because of the involvement of conserved proteins.
Since plants lack adaptive immunity and dedicated immune cells and organs in vertebrates, they may not be as efficient as vertebrates in eliminating viruses during a host shift event. Thus, plants may provide a more conducive environment for a successful host shift event.
5.4. Majority of Plant Viruses are RNA Viruses with High Mutation Rates
Inside a non-host cell, the virus may experience lower fitness resulting in an unsuccessful host shift event. For a successful host shift event, the virus should improve its fitness in the new host. Fitness is a cumulative ability of the virus to utilize the host resources for its (i) multiplication, (ii) ability to counteract the host defense network and (iii) virulence and transmission potential. The majority of plant viruses are RNA viruses [62Joshi S, Haenni LA. Review: Plant RNA viruses: Strategies of expression and regulation of Viral Genes. FEBS Lett 1984; 177.], and these viruses have the highest mutation rates [63Duffy S, Shackelton LA, Holmes EC. Rates of evolutionary change in viruses: Patterns and determinants. Nat Rev Genet 2008; 9(4): 267-76.
[http://dx.doi.org/10.1038/nrg2323] [PMID: 18319742] ]. Mutations can result in variations on which natural selection could act to improve the fitness of the virus [64Zhao L, Duffy S. Gauging genetic diversity of generalists: A test of genetic and ecological generalism with RNA virus experimental evolution. Virus Evol 2019; 5(1)vez019
[http://dx.doi.org/10.1093/ve/vez019] [PMID: 31275611] ]. Therefore, plant viruses can adapt faster during a host shift event. This could also explain the generalist nature of RNA viruses [37Longdon B, Brockhurst MA, Russell CA, Welch JJ, Jiggins FM. The evolution and genetics of virus host shifts. PLoS Pathog 2014; 10(11)e1004395
[http://dx.doi.org/10.1371/journal.ppat.1004395] [PMID: 25375777] ].
5.5. Plasmodesmata in Plants Provides Easy Passage of the Virus for Inter-cellular Movement
After replicating inside a cell, the virus must infect neighbouring (short distance travel) and distantly located (long distance travel) cells in a multicellular organism. However, the virus faces the cell membrane and/or cell wall as barriers while exiting the infected cell and entering a new cell. Transmission of viruses inside an organism can occur either by cell-free mode or by cell-cell connections. In cell-free mode, viruses exit the cell by exocytosis or cell lysis and diffuse to infect the neighbouring or the distantly located cells [65Mothes W, Sherer NM, Jin J, Zhong P. Virus cell-to-cell transmission. J Virol 2010; 84(17): 8360-8.
[http://dx.doi.org/10.1128/JVI.00443-10] [PMID: 20375157] , 66Zhong P, Agosto LM, Munro JB, Mothes W. Cell-to-cell transmission of viruses. Curr Opin Virol 2013; 3(1): 44-50.
[http://dx.doi.org/10.1016/j.coviro.2012.11.004] [PMID: 23219376] ]. In the cell-cell mode of transmission, viruses use cell-to-cell connections like a tight junction, gap junction, cytonemes, plasmodesmata, etc. for their spread. Cell-to-cell transmission is advantageous for the virus since it is faster, protects the virus from the immunological mediators like antibodies and anti-viral factors present in the extracellular space, evades the need for exocytosis or cell lysis and protects the virus from the extracellular environment, which may destabilise viruses [65Mothes W, Sherer NM, Jin J, Zhong P. Virus cell-to-cell transmission. J Virol 2010; 84(17): 8360-8.
[http://dx.doi.org/10.1128/JVI.00443-10] [PMID: 20375157] -67Sherer NM, Mothes W. Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis. Trends Cell Biol 2008; 18(9): 414-20.
[http://dx.doi.org/10.1016/j.tcb.2008.07.003] [PMID: 18703335] ].
The majority of the animal viruses use the cell-free transmission method [49Payne S. Virus Interactions With the Cell.From Understanding to Investigation 2018; 23-35., 68Bird SW, Kirkegaard K. Escape of non-enveloped virus from intact cells. Virology 2015; 479-480: 444-9.
[http://dx.doi.org/10.1016/j.virol.2015.03.044] [PMID: 25890822] ], whereas plant viruses exclusively use a cell-to-cell mode of transmission through plasmodesmata, which are fine cytoplasmic channels running between two plant cells [69Benitez-Alfonso Y, Faulkner C, Ritzenthaler C, Maule AJ. Plasmodesmata: Gateways to local and systemic virus infection. Mol Plant Microbe Interact 2010; 23(11): 1403-12.
[http://dx.doi.org/10.1094/MPMI-05-10-0116] [PMID: 20687788] ]. This is because plant cells have a rigid cell wall and hence viruses cannot carry out a cell-free mode of transmission. Movement through plasmodesmata is assisted by movement proteins, some of which have been found to be conserved across species (Unpublished data). Therefore, plant viruses exploit plasmodesmata to infect neighbouring cells and also enter phloem sieve elements for long distance travel to infect distantly located cells [69Benitez-Alfonso Y, Faulkner C, Ritzenthaler C, Maule AJ. Plasmodesmata: Gateways to local and systemic virus infection. Mol Plant Microbe Interact 2010; 23(11): 1403-12.
[http://dx.doi.org/10.1094/MPMI-05-10-0116] [PMID: 20687788] -71Elisabeth Waigmann SU, Trutnyeva K, Citovsky V. The Ins and Outs of Nondestructive Cell-to-Cell and Systemic Movement of Plant Viruses. Crit Rev Plant Sci 2010; 23: 195-250.
[http://dx.doi.org/10.1080/07352680490452807] ] (Fig. 2c ). Thus, it may be easier for a plant virus to adapt to spread inside the plant during a host shift event than animal viruses.
). Thus, it may be easier for a plant virus to adapt to spread inside the plant during a host shift event than animal viruses.
5.6. Plant Viruses have Access to Different Plant Cell Types and Experience Different Fitness in each Cell Type
The entry of the animal viruses inside the cell is mediated via a specific receptor present on the host cell surface, as explained earlier [51Duffy S, Turner PE, Burch CL. Pleiotropic costs of niche expansion in the RNA bacteriophage phi 6. Genetics 2006; 172(2): 751-7.
[http://dx.doi.org/10.1534/genetics.105.051136] [PMID: 16299384] -53Shimakage MI, Yutsudo M, Hakura A, Toyoshima K. Isolation and characterization of host range mutants of avian sarcoma virus. J Gen Virol 1983; 64(Pt 4): 921-6.
[http://dx.doi.org/10.1099/0022-1317-64-4-921] [PMID: 6300310] ]. Therefore, animal viruses, after entering the organism, find the target tissue(s) and infect specific cell(s) of the tissue(s). Hence, an animal virus can only infect one or a few cell types of the organism [72Samuel Baron MF, Albrecht T. Viral Pathogenesis.Medical Microbiology 1996.]. On the contrary, plant viruses use plasmodesmata for short distance travel to infect neighbouring cells and phloem sieve elements for long distance travel to infect distantly located cells [70Hipper C, Brault V, Ziegler-Graff V, Revers F. Viral and cellular factors involved in Phloem transport of plant viruses. Front Plant Sci 2013; 4: 154.
[http://dx.doi.org/10.3389/fpls.2013.00154] [PMID: 23745125] , 71Elisabeth Waigmann SU, Trutnyeva K, Citovsky V. The Ins and Outs of Nondestructive Cell-to-Cell and Systemic Movement of Plant Viruses. Crit Rev Plant Sci 2010; 23: 195-250.
[http://dx.doi.org/10.1080/07352680490452807] ]. Therefore, plant viruses gain access to different cell types of the plant and often lead to systemic infection [71Elisabeth Waigmann SU, Trutnyeva K, Citovsky V. The Ins and Outs of Nondestructive Cell-to-Cell and Systemic Movement of Plant Viruses. Crit Rev Plant Sci 2010; 23: 195-250.
[http://dx.doi.org/10.1080/07352680490452807] ]. Different cell types of the plant provide different cellular environments to the virus. Thus, plant viruses may experience different fitness in different cell types. This improves the chances of a virus to find the right cell type where the fitness is high and it can multiply and increase the chances of a successful host shift (Fig. 2c ).
).
CONCLUSION
Co-evolution of the virus and the host may result in the virus becoming more specilialised and losing its host(s) [42Woolhouse ME, Taylor LH, Haydon DT. Population biology of multihost pathogens. Science 2001; 292(5519): 1109-12.
[http://dx.doi.org/10.1126/science.1059026] [PMID: 11352066] , 43Whitlock MC. The Red Queen Beats the Jack-Of-All-Trades: The Limitations on the Evolution of Phenotypic Plasticity and Niche Breadth. Am Nat 1996; 148: 565-77.
[http://dx.doi.org/10.1086/285902] ]. However, there are certain advantages to the generalist nature of viruses, and host shift events are common in nature. The majority of the emerging viral diseases are the result of host shift events [8Parrish CR, Holmes EC, Morens DM, et al. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol Mol Biol Rev 2008; 72(3): 457-70.
[http://dx.doi.org/10.1128/MMBR.00004-08] [PMID: 18772285] -10Morens DM, Fauci AS. Emerging infectious diseases: Threats to human health and global stability. PLoS Pathog 2013; 9(7)e1003467
[http://dx.doi.org/10.1371/journal.ppat.1003467] [PMID: 23853589] ]. Examples of other viral diseases resulting from host shift events are SARS, MARS, Avian influenza, Ebola, HIV, etc [8Parrish CR, Holmes EC, Morens DM, et al. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol Mol Biol Rev 2008; 72(3): 457-70.
[http://dx.doi.org/10.1128/MMBR.00004-08] [PMID: 18772285] -10Morens DM, Fauci AS. Emerging infectious diseases: Threats to human health and global stability. PLoS Pathog 2013; 9(7)e1003467
[http://dx.doi.org/10.1371/journal.ppat.1003467] [PMID: 23853589] ]. As discussed above, host shift events in plants are easier to achieve and pose a serious threat to agricultural productivity. However, emerging plant viral diseases do not get the same attention as animal viral diseases and hence there are limited studies in this regard for plant viruses [9Maria R, Rojas RLG. Emerging plant viruses: A diversity of mechanisms and opportunities.Plant Virus Evolution 2008; 27-51.
, 12Hanssen IM, Lapidot M, Thomma BP. Emerging viral diseases of tomato crops. Mol Plant Microbe Interact 2010; 23(5): 539-48.
[http://dx.doi.org/10.1094/MPMI-23-5-0539] [PMID: 20367462] , 13Moriones E, Praveen S, Chakraborty S. Tomato leaf curl new delhi virus: An emerging virus complex threatening vegetable and fiber crops. Viruses 2017; 9(10)E264
[http://dx.doi.org/10.3390/v9100264] [PMID: 28934148] ]. Understanding the mechanism of the host shift events can help us predict the potential hosts of a virus and devise strategies to manage them. Wildlife can act as reservoirs for many zoonotic viruses [73Kruse H, kirkemo AM, Handeland K. Wildlife as source of zoonotic infections. Emerg Infect Dis 2004; 10(12): 2067-72.
[http://dx.doi.org/10.3201/eid1012.040707] [PMID: 15663840] ]. Due to globalisation and the encroachment of humans into the wildlife habitats, diversity of wildlife animals and the viruses and climate change, the emergence of new zoonotic diseases poses a threat to global health. Therefore, research aiming at their discovery, surveillance and understanding should be encouraged [74Carroll D, Watson B, Togami E, et al. Building a global atlas of zoonotic viruses. Bull World Health Organ 2018; 96(4): 292-4.
[http://dx.doi.org/10.2471/BLT.17.205005] [PMID: 29695886] -76Morse SS, Mazet JA, Woolhouse M, et al. Prediction and prevention of the next pandemic zoonosis. Lancet 2012; 380(9857): 1956-65.
[http://dx.doi.org/10.1016/S0140-6736(12)61684-5] [PMID: 23200504] ]. This review is intended to encourage the scientific community to further investigate and understand molecular mechanisms of host shift events of viruses, with the hope that in the near future, the scientific community will be able to predict the future hosts of viruses and be prepared to better cope with their negative effects.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
| [1] | Payne S. Viruses: From Understanding to Investigation 2018. |
| [2] | Roossinck MJ. Lifestyles of plant viruses. Philos Trans R Soc Lond B Biol Sci 2010; 365(1548): 1899-905. [http://dx.doi.org/10.1098/rstb.2010.0057] [PMID: 20478885] |
| [3] | Strauss E, Strauss J. Viruses and Human Disease 2nd ed. 2008. |
| [4] | Payne S. Virus Transmission and Epidemiology.Viruses: From Understanding to Investigation 2018; 53-60. |
| [5] | Whitfield AE, Falk BW, Rotenberg D. Insect vector-mediated transmission of plant viruses. Virology 2015; 479-480: 278-89. [http://dx.doi.org/10.1016/j.virol.2015.03.026] [PMID: 25824478] |
| [6] | Stevens WA. Transmission of Plant Viruses.Virology of Flowering Plants 1983; 41-68. [http://dx.doi.org/10.1007/978-1-4757-1251-3_3] |
| [7] | Elena SF, Agudelo-Romero P, Lalić J. The evolution of viruses in multi-host fitness landscapes. Open Virol J 2009; 3: 1-6. [http://dx.doi.org/10.2174/1874357900903010001] [PMID: 19572052] |
| [8] | Parrish CR, Holmes EC, Morens DM, et al. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol Mol Biol Rev 2008; 72(3): 457-70. [http://dx.doi.org/10.1128/MMBR.00004-08] [PMID: 18772285] |
| [9] | Maria R, Rojas RLG. Emerging plant viruses: A diversity of mechanisms and opportunities.Plant Virus Evolution 2008; 27-51. |
| [10] | Morens DM, Fauci AS. Emerging infectious diseases: Threats to human health and global stability. PLoS Pathog 2013; 9(7)e1003467 [http://dx.doi.org/10.1371/journal.ppat.1003467] [PMID: 23853589] |
| [11] | Ji W, Wang W, Zhao X, Zai J, Li X. 2020.Homologous recombination within the spike glycoprotein of the newly identified coronavirus may boost cross‐species transmission from snake to human. Journal of Medical Virology [http://dx.doi.org/10.1002/jmv.25682] |
| [12] | Hanssen IM, Lapidot M, Thomma BP. Emerging viral diseases of tomato crops. Mol Plant Microbe Interact 2010; 23(5): 539-48. [http://dx.doi.org/10.1094/MPMI-23-5-0539] [PMID: 20367462] |
| [13] | Moriones E, Praveen S, Chakraborty S. Tomato leaf curl new delhi virus: An emerging virus complex threatening vegetable and fiber crops. Viruses 2017; 9(10)E264 [http://dx.doi.org/10.3390/v9100264] [PMID: 28934148] |
| [14] | Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci 2001; 356(1411): 983-9. [http://dx.doi.org/10.1098/rstb.2001.0888] [PMID: 11516376] |
| [15] | Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature 2008; 451(7181): 990-3. [http://dx.doi.org/10.1038/nature06536] [PMID: 18288193] |
| [16] | Luis AD, Hayman DT, O'Shea TJ, et al. 2013; A comparison of bats and rodents as reservoirs of zoonotic viruses: Are bats special? Proceedings Biological sciences 280(1756): 2012-753. [http://dx.doi.org/10.1098/rspb.2012.2753] |
| [17] | Bengis RG, Leighton FA, Fischer JR, Artois M, Mörner T, Tate CM. The role of wildlife in emerging and re-emerging zoonoses. Rev Sci Tech 2004; 23(2): 497-511. [PMID: 15702716] |
| [18] | Rosenberg R. Detecting the emergence of novel, zoonotic viruses pathogenic to humans. Cell Mol Life Sci 2015; 72(6): 1115-25. [http://dx.doi.org/10.1007/s00018-014-1785-y] [PMID: 25416679] |
| [19] | Wang LF, Crameri G. Emerging zoonotic viral diseases. Rev Sci Tech 2014; 33(2): 569-81. [http://dx.doi.org/10.20506/rst.33.2.2311] [PMID: 25707184] |
| [20] | Perlman S. Another decade, another coronavirus. N Engl J Med 2020; 382(8): 760-2. [http://dx.doi.org/10.1056/NEJMe2001126] [PMID: 31978944] |
| [21] | Salata C, Calistri A, Parolin C, Palù G. 2019; Coronaviruses: A paradigm of new emerging zoonotic diseases. Pathogens and disease 77(9): ftaa006. [http://dx.doi.org/10.1093/femspd/ftaa006] |
| [22] | Chua KB, Voon K, Yu M, Keniscope C, Abdul Rasid K, Wang LF. Investigation of a potential zoonotic transmission of orthoreovirus associated with acute influenza-like illness in an adult patient. PLoS One 2011; 6(10)e25434 [http://dx.doi.org/10.1371/journal.pone.0025434] [PMID: 22022394] |
| [23] | Chua KB, Crameri G, Hyatt A, et al. A previously unknown reovirus of bat origin is associated with an acute respiratory disease in humans. Proc Natl Acad Sci USA 2007; 104(27): 11424-9. [http://dx.doi.org/10.1073/pnas.0701372104] [PMID: 17592121] |
| [24] | Chua KB, Voon K, Crameri G, et al. Identification and characterization of a new orthoreovirus from patients with acute respiratory infections. PLoS One 2008; 3(11)e3803 [http://dx.doi.org/10.1371/journal.pone.0003803] [PMID: 19030226] |
| [25] | Dou D, Revol R, Östbye H, Wang H, Daniels R. Influenza a virus cell entry, replication, virion assembly and movement. Front Immunol 2018; 9: 1581. [http://dx.doi.org/10.3389/fimmu.2018.01581] [PMID: 30079062] |
| [26] | Richard M, de Graaf M, Herfst S. Avian influenza a viruses: From zoonosis to pandemic. Future Virol 2014; 9(5): 513-24. [http://dx.doi.org/10.2217/fvl.14.30] [PMID: 25214882] |
| [27] | Watashi K, Wakita T. Hepatitis b virus and hepatitis d virus entry, species specificity, and tissue tropism. Cold Spring Harb Perspect Med 2015; 5(8)a021378 [http://dx.doi.org/10.1101/cshperspect.a021378] [PMID: 26238794] |
| [28] | Srinivasan R, Alvarez JM, Bosque-Pérez NA, Eigenbrode SD, Novy RG. Effect of an alternate weed host, hairy nightshade, Solanum sarrachoides, on the biology of the two most important potato leafroll virus (Luteoviridae: Polerovirus) vectors, Myzus persicae and Macrosiphum euphorbiae (Aphididae: Homoptera). Environ Entomol 2008; 37(2): 592-600. [PMID: 18419933] |
| [29] | Cervantes FA, Alvarez JM. Within plant distribution of Potato Virus Y in hairy nightshade (Solanum sarrachoides): An inoculum source affecting PVY aphid transmission. Virus Res 2011; 159(2): 194-200. [http://dx.doi.org/10.1016/j.virusres.2011.05.003] [PMID: 21601597] |
| [30] | Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Bats: Important reservoir hosts of emerging viruses. Clin Microbiol Rev 2006; 19(3): 531-45. [http://dx.doi.org/10.1128/CMR.00017-06] [PMID: 16847084] |
| [31] | Dawkins R, Krebs JR. Arms races between and within species. Proc R Soc Lond B Biol Sci 1979; 205(1161): 489-511. [http://dx.doi.org/10.1098/rspb.1979.0081] [PMID: 42057] |
| [32] | de Ronde D, Butterbach P, Kormelink R. Dominant resistance against plant viruses. Front Plant Sci 2014; 5: 307. [http://dx.doi.org/10.3389/fpls.2014.00307] [PMID: 25018765] |
| [33] | Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol 2008; 8(11): 889-95. [http://dx.doi.org/10.1038/nri2432] [PMID: 18927577] |
| [34] | Rosenzweig ML, Brown JS, Vincent TL. Red Queens and ESS: The coevolution of evolutionary rates. Evol Ecol 1987; 1: 59-94. [http://dx.doi.org/10.1007/BF02067269] |
| [35] | Anderson RM, May RM. Coevolution of hosts and parasites. Parasitology 1982; 85(Pt 2): 411-26. [http://dx.doi.org/10.1017/S0031182000055360] [PMID: 6755367] |
| [36] | Froissart R, Roze D, Uzest M, Galibert L, Blanc S, Michalakis Y. Recombination every day: Abundant recombination in a virus during a single multi-cellular host infection. PLoS Biol 2005; 3(3)e89 [http://dx.doi.org/10.1371/journal.pbio.0030089] [PMID: 15737066] |
| [37] | Longdon B, Brockhurst MA, Russell CA, Welch JJ, Jiggins FM. The evolution and genetics of virus host shifts. PLoS Pathog 2014; 10(11)e1004395 [http://dx.doi.org/10.1371/journal.ppat.1004395] [PMID: 25375777] |
| [38] | Syverton JT, Berry GP. Multiple virus infection of single host cells. J Exp Med 1947; 86(2): 145-52. [http://dx.doi.org/10.1084/jem.86.2.145] [PMID: 19871662] |
| [39] | De Clercq E. Antiviral agents active against influenza a viruses. Nat Rev Drug Discov 2006; 5(12): 1015-25. [http://dx.doi.org/10.1038/nrd2175] [PMID: 17139286] |
| [40] | Vergara-Alert J, Busquets N, Ballester M, et al. The NS segment of H5N1 Avian Influenza Viruses (AIV) enhances the virulence of an H7N1 AIV in chickens. Vet Res (Faisalabad) 2014; 45: 7. [http://dx.doi.org/10.1186/1297-9716-45-7] [PMID: 24460592] |
| [41] | Reid AH, Taubenberger JK. The origin of the 1918 pandemic influenza virus: A continuing enigma. J Gen Virol 2003; 84(Pt 9): 2285-92. [http://dx.doi.org/10.1099/vir.0.19302-0] [PMID: 12917448] |
| [42] | Woolhouse ME, Taylor LH, Haydon DT. Population biology of multihost pathogens. Science 2001; 292(5519): 1109-12. [http://dx.doi.org/10.1126/science.1059026] [PMID: 11352066] |
| [43] | Whitlock MC. The Red Queen Beats the Jack-Of-All-Trades: The Limitations on the Evolution of Phenotypic Plasticity and Niche Breadth. Am Nat 1996; 148: 565-77. [http://dx.doi.org/10.1086/285902] |
| [44] | Bera S, Fraile A, García-Arenal F. Analysis of fitness trade-offs in the host range expansion of an rna virus, tobacco mild green mosaic virus. J Virol 2018; 92(24): 92. [http://dx.doi.org/10.1128/JVI.01268-18] [PMID: 30257999] |
| [45] | Sharp PM, Hahn BH. The evolution of HIV-1 and the origin of AIDS. Philos Trans R Soc Lond B Biol Sci 2010; 365(1552): 2487-94. [http://dx.doi.org/10.1098/rstb.2010.0031] [PMID: 20643738] |
| [46] | Baronchelli A, Chater N, Christiansen MH, Pastor-Satorras R. Evolution in a changing environment. PLoS One 2013; 8(1)e52742 [http://dx.doi.org/10.1371/journal.pone.0052742] [PMID: 23326355] |
| [47] | Power AG, Flecker AS. Virus Specificity in Disease Systems: Are Species Redundant?The Importance of Species: Perspectives on Expendability and Triage 2003; 330-47. [http://dx.doi.org/10.1515/9781400866779-023] |
| [48] | Bandín I, Dopazo CP. Host range, host specificity and hypothesized host shift events among viruses of lower vertebrates. Vet Res (Faisalabad) 2011; 42(1): 67. [http://dx.doi.org/10.1186/1297-9716-42-67] [PMID: 21592358] |
| [49] | Payne S. Virus Interactions With the Cell.From Understanding to Investigation 2018; 23-35. |
| [50] | Woolhouse M, Scott F, Hudson Z, Howey R, Chase-Topping M. Human viruses: Discovery and emergence. Philos Trans R Soc Lond B Biol Sci 2012; 367(1604): 2864-71. [http://dx.doi.org/10.1098/rstb.2011.0354] [PMID: 22966141] |
| [51] | Duffy S, Turner PE, Burch CL. Pleiotropic costs of niche expansion in the RNA bacteriophage phi 6. Genetics 2006; 172(2): 751-7. [http://dx.doi.org/10.1534/genetics.105.051136] [PMID: 16299384] |
| [52] | Hueffer K, Govindasamy L, Agbandje-McKenna M, Parrish CR. Combinations of two capsid regions controlling canine host range determine canine transferrin receptor binding by canine and feline parvoviruses. J Virol 2003; 77(18): 10099-105. [http://dx.doi.org/10.1128/JVI.77.18.10099-10105.2003] [PMID: 12941920] |
| [53] | Shimakage MI, Yutsudo M, Hakura A, Toyoshima K. Isolation and characterization of host range mutants of avian sarcoma virus. J Gen Virol 1983; 64(Pt 4): 921-6. [http://dx.doi.org/10.1099/0022-1317-64-4-921] [PMID: 6300310] |
| [54] | Balique F, Lecoq H, Raoult D, Colson P. Can plant viruses cross the kingdom border and be pathogenic to humans? Viruses 2015; 7(4): 2074-98. [http://dx.doi.org/10.3390/v7042074] [PMID: 25903834] |
| [55] | Barber GN. Host defense, viruses and apoptosis. Cell Death Differ 2001; 8(2): 113-26. [http://dx.doi.org/10.1038/sj.cdd.4400823] [PMID: 11313713] |
| [56] | Yatim KM, Lakkis FG. A brief journey through the immune system. Clin J Am Soc Nephrol 2015; 10(7): 1274-81. [http://dx.doi.org/10.2215/CJN.10031014] [PMID: 25845377] |
| [57] | Iriti M, Faoro F. Review of innate and specific immunity in plants and animals. Mycopathologia 2007; 164(2): 57-64. [http://dx.doi.org/10.1007/s11046-007-9026-7] [PMID: 17554637] |
| [58] | Turner JG, Ellis C, Devoto A. The jasmonate signal pathway. Plant Cell 2002; 14(Suppl.): S153-64. [http://dx.doi.org/10.1105/tpc.000679] [PMID: 12045275] |
| [59] | Jones JD, Dangl JL. The plant immune system. Nature 2006; 444(7117): 323-9. [http://dx.doi.org/10.1038/nature05286] [PMID: 17108957] |
| [60] | Rosa C, Kuo YW, Wuriyanghan H, Falk BW. RNA interference mechanisms and applications in plant pathology. Annu Rev Phytopathol 2018; 56: 581-610. [http://dx.doi.org/10.1146/annurev-phyto-080417-050044] [PMID: 29979927] |
| [61] | Voinnet O. Induction and suppression of RNA silencing: Insights from viral infections. Nat Rev Genet 2005; 6(3): 206-20. [http://dx.doi.org/10.1038/nrg1555] [PMID: 15703763] |
| [62] | Joshi S, Haenni LA. Review: Plant RNA viruses: Strategies of expression and regulation of Viral Genes. FEBS Lett 1984; 177. |
| [63] | Duffy S, Shackelton LA, Holmes EC. Rates of evolutionary change in viruses: Patterns and determinants. Nat Rev Genet 2008; 9(4): 267-76. [http://dx.doi.org/10.1038/nrg2323] [PMID: 18319742] |
| [64] | Zhao L, Duffy S. Gauging genetic diversity of generalists: A test of genetic and ecological generalism with RNA virus experimental evolution. Virus Evol 2019; 5(1)vez019 [http://dx.doi.org/10.1093/ve/vez019] [PMID: 31275611] |
| [65] | Mothes W, Sherer NM, Jin J, Zhong P. Virus cell-to-cell transmission. J Virol 2010; 84(17): 8360-8. [http://dx.doi.org/10.1128/JVI.00443-10] [PMID: 20375157] |
| [66] | Zhong P, Agosto LM, Munro JB, Mothes W. Cell-to-cell transmission of viruses. Curr Opin Virol 2013; 3(1): 44-50. [http://dx.doi.org/10.1016/j.coviro.2012.11.004] [PMID: 23219376] |
| [67] | Sherer NM, Mothes W. Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis. Trends Cell Biol 2008; 18(9): 414-20. [http://dx.doi.org/10.1016/j.tcb.2008.07.003] [PMID: 18703335] |
| [68] | Bird SW, Kirkegaard K. Escape of non-enveloped virus from intact cells. Virology 2015; 479-480: 444-9. [http://dx.doi.org/10.1016/j.virol.2015.03.044] [PMID: 25890822] |
| [69] | Benitez-Alfonso Y, Faulkner C, Ritzenthaler C, Maule AJ. Plasmodesmata: Gateways to local and systemic virus infection. Mol Plant Microbe Interact 2010; 23(11): 1403-12. [http://dx.doi.org/10.1094/MPMI-05-10-0116] [PMID: 20687788] |
| [70] | Hipper C, Brault V, Ziegler-Graff V, Revers F. Viral and cellular factors involved in Phloem transport of plant viruses. Front Plant Sci 2013; 4: 154. [http://dx.doi.org/10.3389/fpls.2013.00154] [PMID: 23745125] |
| [71] | Elisabeth Waigmann SU, Trutnyeva K, Citovsky V. The Ins and Outs of Nondestructive Cell-to-Cell and Systemic Movement of Plant Viruses. Crit Rev Plant Sci 2010; 23: 195-250. [http://dx.doi.org/10.1080/07352680490452807] |
| [72] | Samuel Baron MF, Albrecht T. Viral Pathogenesis.Medical Microbiology 1996. |
| [73] | Kruse H, kirkemo AM, Handeland K. Wildlife as source of zoonotic infections. Emerg Infect Dis 2004; 10(12): 2067-72. [http://dx.doi.org/10.3201/eid1012.040707] [PMID: 15663840] |
| [74] | Carroll D, Watson B, Togami E, et al. Building a global atlas of zoonotic viruses. Bull World Health Organ 2018; 96(4): 292-4. [http://dx.doi.org/10.2471/BLT.17.205005] [PMID: 29695886] |
| [75] | King DA, Peckham C, Waage JK, Brownlie J, Woolhouse ME. Epidemiology. Infectious diseases: Preparing for the future. Science 2006; 313(5792): 1392-3. [http://dx.doi.org/10.1126/science.1129134] [PMID: 16959992] |
| [76] | Morse SS, Mazet JA, Woolhouse M, et al. Prediction and prevention of the next pandemic zoonosis. Lancet 2012; 380(9857): 1956-65. [http://dx.doi.org/10.1016/S0140-6736(12)61684-5] [PMID: 23200504] |





