- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Synthesis and Evaluation of New 2-Iminothiazolidin-4-one and Thiazolidin-2,4-dione Derivatives as Antimicrobial and Anti-inflammatory Agents
Waleed A. Bayoumi1, *, Shaymaa H. Abdel-Rhman2, Mohamed E. Shaker3
Abstract
A new series of 2-iminothiazolidin-4-ones and thiazolidin-2,4-diones were designed and synthesized. The target compounds were evaluated for antimicrobial and anti-inflammatory activities, aiming to find a candidate carrying dual activities. The antibacterial activity was determined by cup-diffusion technique and NCCLS broth dilution method. The MICs were determined using 96-well microtitre plates, while the anti-inflammatory activity was determined using carrageenan- induced rat paw edema model. The results revealed that target compounds showed no antifungal activity towards tested Fungi, whereas the antibacterial activity was mainly on Gram-positive bacteria. The anti-inflammatory activity was observed in compounds belonging to the thiazolidin-2,4-dione series. The structures of the newly synthesized compounds were confirmed by physical and spectral data.
Article Information
Identifiers and Pagination:
Year: 2014Volume: 1
First Page: 33
Last Page: 38
Publisher Id: CHEM-1-33
DOI: 10.2174/1874842201401010033
Article History:
Received Date: 24/9/2014Revision Received Date: 26/11/2014
Acceptance Date: 27/11/2014
Electronic publication date: 31/12/2014
Collection year: 2014
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Department of Pharmaceutical Organic Chemistry, Faculty of Pharmacy, University of Mansoura, Mansoura 35516, Egypt; Tel: +20 111 5633 722; Fax: +20 5022 4749; E-mail: waleedbayoumi@mans.edu.eg
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 24-9-2014 |
Original Manuscript | Synthesis and Evaluation of New 2-Iminothiazolidin-4-one and Thiazolidin-2,4-dione Derivatives as Antimicrobial and Anti-inflammatory Agents | |
INTRODUCTION
The search for new anti-inflammatory agents devoid of potential undesirable side effects and new antimicrobial agents capable of overcoming the problem of increasing microbial resistance continues to be an active research area in pharmaceutical chemistry. Of the most interesting heterocyclic scaffolds available for modulation of biological activity are: thiazolidinone and thiazolidindione. Their different biological activities were reported [1Jain, A K; Vaidya, A; Ravichandran, V; Kashaw, S K; Agrawal, R K Recent developments and biological activities of thiazolidinone derivatives: A review Bioorg. Med. Chem, 2012, 20, 3378-3395., 2Abhinit, M; Ghodke, M; Pratima, N A Exploring potential of 4-thiazolidinone: a brief review Int. J. Pharm. Pharm. Sci, 2009, 1, 47-64.]. Among the most important biological and pharmacological activities of both heterocyclic pharmacophoric moieties are: antihyperglycemic [3Raza, S; Srivastava, S P; Srivastava, D S; Srivastava, A K; Haq, W; Katti, S B Thiazolidin-4-one and thiazinan-4-one derivatives analogous to rosiglitazone as potential antihyperglycemic and antidyslipidemic agents Eur. J. Med. Chem, 2013, 63, 611-620., 4Lee, H W; Kim, B Y; Ahn, J B; Kang, S W; Lee, J H; Shin, J S; Ahn, S K; Lee, S J; Yoon, S S Molecular design, synthesis, and hypoglycemic and hypolipidemic activities of novel pyrimidine derivatives having thiazolidinedione Eur. J. Med. Chem, 2005, 40, 862-874.
[http://dx.doi.org/10.1016/j.ejmech.2005.03.019] ], antibacterial [5Kunzler, A; Neuenfeldt, P D; das Neves, A M; Pereira, C M P; Marques, G H; Nascente, P S; Fernandes, M H V; Hübner, S O; Cunico, W Synthesis, antifungal and cytotoxic activities of 2-aryl-3-((piperidin-1-yl)ethyl)thiazolidinones Eur. J. Med. Chem, 2013, 64, 74-80.-9Sattigeri, V J; Son, A; Singhal, S; Khan, S; Pandya, M; Bhateja, P; Mathur, T; Rattan, A; Khanna, J M; Mehta, A Synthesis and antimicrobial activity of novel thiazolidinones ARKIVOC, 2005, ii, 46-59.], antifungal [10Omar, K; Geronikaki, A; Zoumpoulakis, P; Camoutsis, C; Soković, M; Ćirić, A; Glamočlija, J Novel 4-thiazolidinone derivatives as potential antifungal and antibacterial drugs Bioorg. Med. Chem, 2010, 18, 426-432., 11Mori, M; Takagi, M; Noritake, C; Kagabu, S 2,4-Dioxo-1,3-thiazolidine derivatives as a lead for new fungicides J. Pestic. Sci, 2008, 33, 357-363.
[http://dx.doi.org/10.1584/jpestics.G08-15] ], anti-inflammatory [12Vazzana, I; Terranova, E; Mattioli, F; Sparatore, F Aromatic Schiff bases and 2,3-disubstituted-1,3-thiazolidin-4-one derivatives as antiinflammatory agents ARKIVOC, 2004, v, 364-374.
[http://dx.doi.org/10.3998/ark.5550190.0005.531] -17Goel, B; Ram, T; Tyagi, R; Bansal, E; Kumar, A; Mukherjee, D; Sinha, J N 2-Substituted-3-(4-bromo-2-carboxyphenyl)-5-methyl-4-thiazolidinones as potential anti-inflammatory agents Eur. J. Med. Chem, 1999, 34, 265-269.
[http://dx.doi.org/10.1016/S0223-5234(99)80060-9] ], cyclooxygenase and lipoxygenase inhibitors [18Geronikaki, A A; Lagunin, A A; Hadjipablou-Litina, D I; Eleftheriou, E T; Filimonov, D A; Poroikov, VV; Alam, I; Saxena, A A Computer-aided discovery of anti-inflammatory thiazolidinones with dual cyclooxygenase/lipoxygenase inhibition J. Med. Chem, 2008, 51, 1601-1609., 19Wu, Y; Karna, S; Choi, CH; Tong, M; Tai, H H; Na, D H; Jang, C H; Cho, H Synthesis and biological evaluation of novel thiazolidinedione analogues as 15-hydroxyprostaglandin dehydrogenase inhibitors J. Med. Chem, 2011, 54, 5260-5264.
[http://dx.doi.org/10.1021/jm200390u] ], anticonvulsants [20Ergenc, N; Capan, G Synthesis and anticonvulsant activity of new 4-thiazolidone and 4-thiazoline derivatives IL Farmaco, 1994, 49, 133-135., 21Dwivedi, C; Gupta, T K; Parmar, S S Substituted thiazolidones as anticonvulsants J. Med. Chem, 1972, 15, 553-554.
[http://dx.doi.org/10.1021/jm00275a031] ], antitumor [22Hafez, H N; El-Gazzar, A R Synthesis and antitumor activity of substituted triazolo[4,3-a]pyrimidin-6-sulfonamide with an incorporated thiazolidinone moiety Bioorg. Med. Chem. Lett, 2009, 19, 4143-4147.
[http://dx.doi.org/10.1016/j.bmcl.2009.05.126] ], antioxidant [23Reddy, K A; Lohray, B B; Bhushan, V; Reddy, A S; Kishore, P H; Rao, V V; Saibaba, V; Bajji, A C; Rajesh, B M; Reddy, K V; Chakrabarti, R; Rajagopalan, R Novel euglycemic and hypolipidemic agents: Part-2 antioxidant moiety as structural motif Bioorg. Med. Chem. Lett, 1998, 8, 999-1002.
[http://dx.doi.org/10.1016/S0960-894X(98)00159-0] ] and several other activities [1Jain, A K; Vaidya, A; Ravichandran, V; Kashaw, S K; Agrawal, R K Recent developments and biological activities of thiazolidinone derivatives: A review Bioorg. Med. Chem, 2012, 20, 3378-3395., 2Abhinit, M; Ghodke, M; Pratima, N A Exploring potential of 4-thiazolidinone: a brief review Int. J. Pharm. Pharm. Sci, 2009, 1, 47-64.].
Those reported variable and numerous pharmacological activities encouraged us to design and synthesize lead molecules of thiazolidinone and thiazolidindione pharmacophores carrying potential antimicrobial and anti-inflammatory activities. Thus, the primary aim of this research is to find newer generations possessing more potent and safe therapeutic profile.
Structure diversity was accomplished by introducing different (un)substituted arylidene functionality at the 5-position of the two core skeletons 2-iminothiazol-4-ones (4a-4d) and thiazolidin-2,4-diones (7a-7d). This arylidene side arm was designed to act as potential bioactive moiety imparting a modification in certain physicochemical properties e.g., lipophilicity and steric effect [24Vicini, P; Zani, F; Cossini, P; Doytchinova, I Hydrazones of 1,2-benzisothiazole hydrazides: synthesis, antimicrobial activity and QSAR investigations Eur. J. Med. Chem, 2002, 37, 553-564.
[http://dx.doi.org/10.1016/S0223-5234(02)01378-8] -26Chavan, A A; Pai, N R Synthesis and antimicrobial screening of 5-arylidene-2-imino-4-thiazolidinones ARKIVOK, 2007, xvi, 148-155.
[http://dx.doi.org/10.3998/ark.5550190.0008.g16] ].
In addition, a benzenesulfonamide moiety was introduced at the 3-position of both skeletons; directly in 4a-4d and via an acetamide linker in 7a-7d. The benzenesulfonamide moiety was reported to be important for potentiation of both antimicrobial and anti-inflammatory activities [27Sharma, P K; Chandak, N; Kumar, P; Sharma, C; Aneja, K R Synthesis and biological evaluation of some 4-functionalized-pyrazoles as antimicrobial agents Eur. J. Med. Chem, 2011, 46, 1425-1432.
[http://dx.doi.org/10.1016/j.ejmech.2011.01.060] -30Alsughayer, A; Elassar, A A; Mustafa, S; Al Sagheer, F Synthesis, structure analysis and antibacterial activity of new potent sulfonamide derivatives J. Biomat. Nanobiotech, 2011, 2, 144-149.
[http://dx.doi.org/10.4236/jbnb.2011.22018] ]. Target skeletons and their structural modifications are illustrated in Fig. (1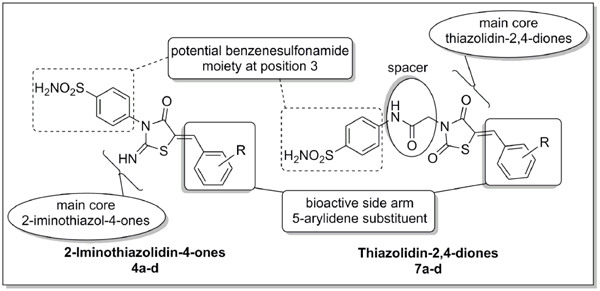 ).
).
 |
Fig. (1) Design of target 2-iminothiazol-4-ones (4a-4d) and thiazolidin-2,4-diones (7a-7d). |
EXPERIMENTAL
Chemistry
Melting points were determined in a capillary tube using Electrothermal C14500 apparatus and are uncorrected. NMR spectra were recorded on a Bruker AC spectrometer (400 MHz-1H and 100.0 MHz-13C) in DMSO-d6. Chemical shifts in ppm are given as δ values against tetramethylsilane as the internal standard. MS analyses were performed on Thermo Fisher Scientific Trace DSQ II, Mass spectrometer at Central Research Lab, Faculty of Pharmacy, Mansoura University. Elemental Microanalyses were performed by the microanalytical centre, Cairo University. Reactions were monitored by TLC performed on silica gel plates (Merck 60 F245) and using ethyl acetate: petroleum ether (1:1) as eluent, the spots were visualized by U.V (366, 245 nm). Compounds 5 [31Sohda, T; Mizuno, K; Imamiya, E; Sugiyama, Y; Fujita, T; Kawamasu, Y Studies on Antidiabetic Agents. II. Synthesis of 5-[4-(1-Methylcyclohexylmethoxy)-benzyl]thiazolidine-2,4-dione (ADD-3878) and its derivatives Chem. Pharm. Bull, 1982, 30, 3580-3600.], 6a-6d [32Ha, YM; Park, YJ; Kim, J; Park, D; Park, J Y; Lee, H J; Lee, J Y; Moon, H R; Chung, H Y Design and synthesis of 5-(substituted benzylidene)thiazolidine-2,4-dione derivatives as novel tyrosinase inhibitors Eur. J. Med. Chem, 2012, 49, 245-252.] were prepared according to the reported procedures.
2-Chloro-N-(4-sulfamoylphenyl)acetamide (1)
Chloroacetyl chloride (8 mL) was added slowly over a period of 30 min to a solution of sulfanilamide (17.2 g, 100 mmol) in DMF (50 mL). Stirring was continued for 1 h. The reaction mixture was quenched with cold water (150 mL). The formed precipitate was collected by filtration, washed with cold water, dried and recrystallized from methanol to afford pure product. Colorless crystals, yield 85%, mp 218-220 °C [33Jacobs, W A; Heidelberger, M J On amides, uramino compounds, and ureides containing an aromatic nucleus J. Am. Chem. Soc, 1917, 39, 2418-2443.
[http://dx.doi.org/10.1021/ja02256a021] ].
4-(2-Imino-4-oxothiazolidin-3-yl)benzenesulfonamide (3)
A mixture of 1 (2.48 g, 10 mmol) and NH4SCN (1.52 g, 20 mmol) in acetone (25 mL) was refluxed for 2 h. The formed precipitate was filtered and dried. The dried solid product was then heated with DMF (20 mL) at 120-130 °C for 1 h. the reaction mixture was cooled to rt and poured over crushed ice to afford white crystalline product, which was filtered, dried and recrystallized from ethanol to afford pure product. White crystals, yield 70%, mp 258-260 °C (reported mp 258 °C) [34Taniyama, H; Takemura, S Studies on thiazole derivatives. I. Synthesis of 4-thiazolidones Yakugaku Zasshi, 1953, 73, 164-165.].
Synthesis of (Z)-4-(2-Imino-5-((un)substituted benzylidene)-4-oxothiazolidin-3-yl)benzenesulfonamides (4a-4d)
General procedure:
Method 1; a mixture of 3 (2.71 g, 10 mmol), the appropriate aromatic aldehyde (10 mmol) and catalytic amount of pyridine in xylene (25 mL) was heated at reflux for 6 h. After cooling to rt, the formed precipitate was filtered, dried and recrystallized from isopropyl alcohol to afford pure products. Method 2; a mixture of 3 (2.71 g, 10 mmol), the appropriate aromatic aldehyde (10 mmol) and catalytic amount of sodium acetate in glacial acetic acid (25 mL) was heated at reflux for 5 h. After cooling to rt, the formed precipitate was filtered, washed thoroughly with cold water, dried and recrystallized from isopropyl alcohol to afford pure products.
(Z)-4-(5-Benzylidene-2-imino-4-oxothiazolidin-3-yl)benzenesulfonamide (4a)
Yellowish white crystals, mp 243-45 °C, yield 60% (method 1), 65% (method 2). Analysis for C16H13N3O3S2 (359.42), Calcd.: C, 53.47; H, 3.65; N, 11.69; Found: C, 53.52 ; H, 3.59; N, 11.74. 1H NMR (DMSO-d6): δ 9.96 (s, 1H, NH, D2O exchangeable), 7.96 (d, 2H, J=8.8 Hz, H-3 and H-5), 7.83 (s, 1H, C=CH), 7.74-7.57 (m, 3H, H-3’, H-4’ and H-5’), 7.52 (d, 2H, J=6.4 Hz, H-2 and H-6), 7.33 (s, 2H, SO2NH2, D2O exchangeable), 7.22 (d, 2H, J=7.7 Hz, H-2’ and H-6’). 13C NMR (DMSO-d6): δ 171.9, 165.7, 141.0, 140.2, 132.8, 132.3, 130.1, 129.4, 128.8, 127.3, 126.8, 120.4. MS m/z (% Rel. Int.): 359 (24.56, M+).
(Z)-4-(2-Imino-5-(4-methoxybenzylidene)-4-oxothiazolidin-3-yl)benzenesulfonamide (4b)
Yellow crystals, mp 256-58 °C, yield 75% (method 1), 75% (method 2). Analysis for C17H15N3O4S2 (389.45), Calcd.: C, 52.43; H, 3.88; N, 10.79; Found: C, 52.50 ; H, 3.72; N, 10.71. 1H NMR (DMSO-d6): δ 9.88 (s, 1H, NH, D2O exchangeable), 7.95 (d, 2H, J=8.4 Hz, H-3 and H-5), 7.83 (s, 1H, C=CH), 7.62 (d, 1H, J=8.4 Hz, H-3’), 7.58 (d, 1H, J=8.4 Hz, H-5’), 7.51 (d, 2H, J=7.6 Hz, H-2 and H-6), 7.33 (s, 2H, SO2NH2, D2O exchangeable), 7.14 (d, 2H, J=8.8 Hz, H-2’ and H-6’), 3.93 (s, 3H, OCH3). 13C NMR (DMSO-d6): δ 170.3, 167.5, 161.6, 141.4, 141.3, 131.9, 130.8, 129.6, 127.9, 125.8, 120.9, 114.3, 56.0. MS m/z (% Rel. Int.): 389 (7.50, M+).
(Z)-4-(5-(3,4-Dimethoxybenzylidene)-2-imino-4-oxothiazolidin-3-yl)benzenesulfonamide (4c)
Yellow crystals, mp 237-39 °C, yield 71% (method 1), 68% (method 2). Analysis for C18H17N3O5S2 (419.47), Calcd.: C, 51.54; H, 4.08; N, 10.02; Found: C, 51.60 ; H, 3.99; N, 10.08. 1H NMR (DMSO-d6): δ 9.88 (s, 1H, NH, D2O exchangeable), 7.95 (d, 2H, J=7.6 Hz, H-3 and H-5), 7.83 (s, 1H, C=CH), 7.69 (d, 1H, J=10.8 Hz, H-6’), 7.62 (d, 1H, J=8 Hz, H-5’), 7.52 (d, 2H, J=7.2 Hz, H-2 and H-6), 7.33 (s, 2H, SO2NH2, D2O exchangeable), 7.25 (s, 1H, H-2’), 3.95 (s, 6H, 2 OCH3). 13C NMR (DMSO-d6): δ 170.8, 163.9, 152.1, 149.1, 141.9, 140.8, 130.3, 130.2, 128.3, 126.5, 124.1, 120.6, 113.4, 112.2, 56.8. MS m/z (% Rel. Int.): 419 (4.39, M+).
(Z)-4-(5-(2-Chloro-5-nitrobenzylidene)-2-imino-4-oxothiazolidin-3-yl)benzenesulfonamide (4d)
Orange crystals, mp 191-193 °C, yield 63% (method 1), 65% (method 2). Analysis for C16H11ClN4O5S2 (438.87), Calcd.: C, 43.79; H, 2.53; N, 12.77; Found: C, 43.65; H, 2.62; N, 12.66. 1H NMR (DMSO-d6): δ 10.27 (s, 1H, NH, D2O exchangeable), 8.38 (s, 1H, H-6’), 8.30 (d, 2H, J=8.4 Hz, H-3 and H-5), 8.18 (s, 1H, C=CH), 7.98 (d, 1H, J=8.4 Hz, H-4’), 7.66 (d, 2H, J=8.4 Hz, H-2 and H-6), 7.53 (s, 2H, SO2NH2, D2O exchangeable), 7.40 (d, 1H, J=7.6 Hz, H-3’). 13C NMR (DMSO-d6): δ 172.7, 164.6, 145.4, 142.1, 140.9, 140.2, 131.5, 130.8, 129.9, 128.6, 126.4, 125.2, 123.4, 120.7. MS m/z (% Rel. Int.): 438 (6.50, M+).
Synthesis of (Z)-2-(5-(un)substituted benzylidene-2,4-dioxothiazolidin-3-yl)-N-(4-sulfamoylphenyl)acetamides (7a-7d)
General procedure: a mixture of 1 (2.49 g, 10 mmol), the appropriate 6a-6d (10 mmol) and K2CO3 (2 g) in DMF (25 mL) was stirred overnight at rt. Quenching with ice-cold water (50 mL) precipitated the product, which was filtered, dried and recrystallized from DMF:water (1:1) mixture to afford pure products.
(Z)-2-(5-Benzylidene-2,4-dioxothiazolidin-3-yl)-N-(4-sulfamoylphenyl)acetamide (7a)
Yellow crystals, mp 250-252 °C, yield 60%. Analysis for C18H15N3O5S2 (417.46), Calcd.: C, 51.79; H, 3.62; N, 10.07; Found: C, 51.68 ; H, 3.70; N, 10.11. 1H NMR (DMSO-d6): δ 10.73 (s, 1H, NH, D2O exchangeable), 8.02 (s, 1H, C=CH), 7.81 (d, 2H, J=8 Hz, H-3 and H-5), 7.75 (d, 2H, J=7.6 Hz, H-2 and H-6), 7.60 (d, 2H, J=7.6 Hz, H-2’ and H-6’), 7.58-7.53 (m, 3H, H-3’, H-4’ and H-5’), 7.31 (s, 2H, SO2NH2, D2O exchangeable), 4.59 (s, 2H, CH2). 13C NMR (DMSO-d6): δ 180.2, 167.1, 165.7, 142.7, 139.3, 132.8, 130.6, 130.1, 129.4, 128.8, 127.9, 120.4, 114.6. 45.9. MS m/z (% Rel. Int.): 417 (26.11, M+).
(Z)-2-(5-(4-Methoxybenzylidene)-2,4-dioxothiazolidin-3-yl)-N-(4-sulfamoylphenyl)acetamide (7b)
Yellow crystals, mp 224-226 °C, yield 68%. Analysis for C19H17N3O6S2 (447.48), Calcd.: C, 51.00; H, 3.83; N, 9.39; Found: C, 51.04; H, 3.78; N, 9.42. 1H NMR (DMSO-d6): δ 10.76 (s, 1H, NH, D2O exchangeable), 7.97 (s, 1H, C=CH), 7.80 (d, 2H, J=8 Hz, H-3 and H-5), 7.73 (d, 2H, J=8.4 Hz, H-2 and H-6), 7.65 (d, 2H, J=8 Hz, H-2’ and H-6’), 7.29 (s, 2H, SO2NH2, D2O exchangeable), 7.14 (d, 2H, J=8.4 Hz, H-3’ and H-5’), 4.59 (s, 2H, CH2), 3.86 (s, 3H, OCH3). 13C NMR (DMSO-d6): δ 180.2, 167.1, 165.7, 161.6, 142.7, 139.3, 130.8, 130.6, 127.9, 125.9, 120.4, 114.6, 114.3, 56.0, 45.9. MS m/z (% Rel. Int.): 447 (22.60, M+).
(Z)-2-(5-(3,4-Dimethoxybenzylidene)-2,4-dioxothiazolidin-3-yl)-N-(4-sulfamoylphenyl)acetamide (7c)
Pale yellow crystals, mp 205-207 °C, yield 65%. Analysis for C20H19N3O7S2 (477.51), Calcd.: C, 50.31; H, 4.01; N, 8.80; Found: C, 50.38; H, 4.95; N, 8.87. 1H NMR (DMSO-d6): δ 10.57 (s, 1H, NH, D2O exchangeable), 7.94 (s, 1H, C=CH), 7.82 (d, 2H, J=7.6 Hz, H-3 and H-5), 7.72 (d, 2H, J=8.8 Hz, H-2 and H-6), 7.27 (d, 1H, J=8 Hz, H-6’), 7.16 (s, 1H, H-2’), 7.17 (d, 1H, J=8 Hz, H-5’), 7.04 (s, 2H, SO2NH2, D2O exchangeable), 4.58 (s, 2H, CH2), 3.87 (s, 6H, 2 OCH3). 13C NMR (DMSO-d6): δ 180.2, 167.1, 165.7, 152.1, 149.1, 142.7, 139.3, 129.4, 127.9, 126.5, 124.1, 120.4, 113.9, 113.4, 112.5, 56.8, 45.9. MS m/z (% Rel. Int.): 477 (21.00, M+).
(Z)-2-(5-(2-Chloro-5-nitrobenzylidene)-2,4-dioxothiazolidin-3-yl)-N-(4-sulfamoylphenyl)acetamide (7d)
Pale yellow crystals, mp 212-214 °C, yield 55%. Analysis for C18H13ClN4O7S2 (496.90), Calcd.: C, 43.51; H, 2.64; N, 11.28; Found: C, 43.58; H, 2.70; N, 11.19. 1H NMR (DMSO-d6): δ 10.59 (s, 1H, NH, D2O exchangeable), 8.20 (s, 1H, H-6’), 8.01 (d, 1H, J=7.6 Hz, H-6’), 7.90 (s, 1H, C=CH), 7.79 (d, 2H, J=7.6 Hz, H-3 and H-5), 7.69 (d, 2H, J=8.8 Hz, H-2 and H-6), 7.51 (d, 1H, J=7.6 Hz, H-3’), 7.04 (s, 2H, SO2NH2, D2O exchangeable), 4.55 (s, 2H, CH2). 13C NMR (DMSO-d6): δ 180.2, 167.1, 165.7, 145.4, 142.7, 140.3, 139.3, 131.5, 130.8, 127.9, 125.9, 125.7, 120.6, 120.4, 117.1, 45.9. MS m/z (% Rel. Int.): 496 (13.44, M+).
Antimicrobial screening
The antimicrobial activity of the synthesized compounds has been studied using the cup-diffusion technique [35Wayne, P Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed., Approved standard M7- A5. National Committee for Clinical Laboratory Standards, vol. 20, 2000.]. The tested compounds were screened against Staphylococcus aureus and Bacillus subtilis as examples for Gram-positive bacteria, Escherichia coli and Pseudomonas aeruginosa as examples for Gram-negative bacteria and Candida albicans and Candida tropicalis as example for fungi.
In this technique pores were made using a sterile cork borer in the solidified agar medium and an aliquot of 0.05 mL of 1000 μg/mL of the tested substance is placed in each pore in the nutrient agar medium on which a culture of the tested bacteria has been spread to produce uniform growth. After 24 h incubation at 37 °C, the diameter of inhibition zone is measured in mm.
Compounds that exhibited good anti-Gram-positive bacteria were assayed in vitro against Staphylococcus aureus and Bacillus subtilis. The organisms were obtained from the Microbiology Laboratory, Department of Microbiology, Faculty of Pharmacy, University of Mansoura. The antibacterial assay was carried out using NCCLS broth dilution method [36Pelczar, M J; Chan, E C S; Krieg, N R Microbiology Concepts and Applications, 6th ed; McGraw-Hill Inc: New York, 1999. , 37Collee, J G; Fraser, A G; Marmion, B P; Simmons, A Mackie and McCartney In: Practical medical microbiology, 14th ed; Churchill Livingstone: London, 1996. ]. The minimum inhibitory concentrations (MICs) were determined using 96-well microtitre plates. The bacterial suspension was adjusted with sterile saline to a concentration of 1.0x105 c.f.u./mL. Compounds to be investigated were dissolved in DMSO. The microplates were incubated for 24 h at 37 °C. In order to ensure that the solvent had no effect on bacterial growth, a control test was performed containing broth supplemented with only DMSO. The lowest concentrations without visible growth were defined as concentrations that completely inhibited bacterial growth (MICs).
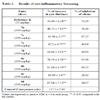
Ampicillin was used as a reference standard to compare the antibacterial activity. All experiments were performed in duplicate and repeated three times. The MIC values were expressed in µg/mL and the results of the most active compounds are shown in Table 1.
Anti-inflammatory Screening
Carrageenan-induced rat paw edema assay
The anti-inflammatory activity of the test compounds was carried out using carrageenan-induced rat paw edema model [38Winter, C A; Risley, E A; Nuss, G W Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs Proc. Soc. Exp. Biol. Med, 1962, 111, 544-547.
[http://dx.doi.org/10.3181/00379727-111-27849] ] by employing carrageenan solution as the phlogistic agent. Male Wistar albino rats weighing 150–180 g were kept in the animal house under standard conditions of light and temperature with free access to food and water. After overnight fasting, the animals were randomly divided into groups each consisting of six rats. The first group served as a control and received only the vehicle (1 mL/kg, i.p., 10% v/v of DMSO in normal saline). Another group received the standard drug Diclofenac K (10 mg/kg, i.p., 1% w/v the vehicle). Other groups of rats were administered the test compounds in equal doses (100 mg/kg, i.p.; w/v in 10% w/v the vehicle). Thirty minutes after administration of the standard drug and test compounds, 0.1 mL of 1% w/v of λ-carrageenan (Type IV, Sigma-Aldrich, USA) suspension in sterile normal saline was injected into the subplanter region of the right hind paw of each rat. After 4 hr, the paw thickness was measured with electronic digital calipers, and the difference in the paw thickness of between the injected and the control was recorded for each rat. The edema was expressed as the percentage of increase in the paw thickness, and the percentage of inhibition of edema for each group.
Statistical analysis
Data obtained from animal experiments were expressed as mean ± standard error (S.E.M.). The statistical significance of difference between groups were determined by means of analysis of variance (ANOVA) followed by Dunnet’s test. The results of the anti-inflammatory studies have been presented in Table 2.
RESULTS AND DISCUSSION
Chemistry
The synthetic methods used for preparation of target compounds are depicted in Schemes 1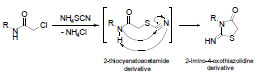 -5
-5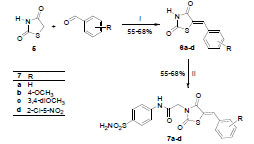 . Compound 1, the precursor of the starting compound was prepared by chloroacetylation of sulfanilamide using chloroacetyl chloride in DMF as a solvent. The starting compound, 3 was prepared through a two-steps reaction. The first step is a nucleophilic substitution of NH4SCN with 1 using acetone as a solvent to afford the intermediate N-(4-sulfamoylphenyl)-2-thiocyanatoacetamide 2, followed by thermal cyclization using DMF as a polar aprotic solvent. The postulated mechanism for heterocyclic cyclization is illustrated (Scheme 1
. Compound 1, the precursor of the starting compound was prepared by chloroacetylation of sulfanilamide using chloroacetyl chloride in DMF as a solvent. The starting compound, 3 was prepared through a two-steps reaction. The first step is a nucleophilic substitution of NH4SCN with 1 using acetone as a solvent to afford the intermediate N-(4-sulfamoylphenyl)-2-thiocyanatoacetamide 2, followed by thermal cyclization using DMF as a polar aprotic solvent. The postulated mechanism for heterocyclic cyclization is illustrated (Scheme 1 ). The synthesis of compound 3 was reported earlier to proceed in a one-step reaction using ethanol or acetone as solvents, but it was observed that the purity of the product was better when using the two-steps synthesis (Scheme 2
). The synthesis of compound 3 was reported earlier to proceed in a one-step reaction using ethanol or acetone as solvents, but it was observed that the purity of the product was better when using the two-steps synthesis (Scheme 2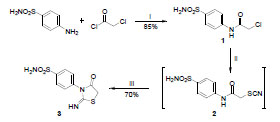 ).
).
 |
Scheme 1 Postulated mechanism for formation of compound 3. |
 |
Scheme 2 Synthesis of compound 3. Reagents and conditions: i) DMF, stirr, rt, 1 h; ii) NH4SCN, acetone, reflux, 2 h; iii) DMF, 120-130 °C, 1 h. |
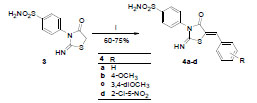 |
Scheme 3 Synthesis of target compounds 4a-4d. Reagents and conditions: i) method 1: ArCHO, xylene, pyridine, reflux, 6 h; method 2: ArCHO, AcOH, AcONa, reflux, 3 h. |
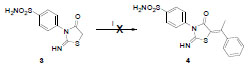 |
Scheme 4 Unsuccessful trials for synthesis of compound 4. Reagents and conditions: i) method 1: acetophenone, xylene, pyridine, reflux, NR; method 2: acetophenone, AcOH, AcONa, NR. |
 |
Scheme 5 Synthesis of target compounds 7a-7d. Reagents and conditions: i) AcOH, AcONa, reflux, 12 h; ii) 1, K2CO3, DMF, rt. |
Synthesis of target compounds, 4a-4d was achieved through Knoevenagel condensation of 3 with the different aromatic aldehydes. The reaction was carried out in two different solvents; in xylene using catalytic amount of pyridine (method 1) and in glacial acetic acid using sodium acetate as catalyst (method 2). Concerning the yield of the two methods, no significant difference was observed, however the purity of the products using method 2 was found to be better (Scheme 3 ).
).
Trial for obtaining Knoevenagel condensation product 4, using acetophenone as a ketone, was found unsuccessful, using both methods at different reflux times (Scheme 4 ).
).
The benzylidenethiazolidine-2,4-diones (6a-6d) were prepared by condensing thiazolidine-2,4-dione (5) [31Sohda, T; Mizuno, K; Imamiya, E; Sugiyama, Y; Fujita, T; Kawamasu, Y Studies on Antidiabetic Agents. II. Synthesis of 5-[4-(1-Methylcyclohexylmethoxy)-benzyl]thiazolidine-2,4-dione (ADD-3878) and its derivatives Chem. Pharm. Bull, 1982, 30, 3580-3600.] with different aromatic aldehydes in glacial acetic acid and using catalytic amount of sodium acetate. Alkylation of 6a-6d with 1 in DMF in presence of K2CO3 at rt afforded the target thiazolidin-2,4-dione derivatives 7a-7d in satisfied yields (Scheme 5 ).
).
Target compounds 4a-4d and 7a-7d can potentially exist in either E or Z geometrical isomeric forms at the 5-exocyclic double bond. The Z configuration was postulated due to the deshielded methine proton signals by adjacent carbonyl group for both 4a-4d (δ 7.83-8.18) and 7a-7d (δ 7.90-8.02) in the 1H NMR spectra. These higher chemical shifts were in coordination with the Z configuration in comparison with the E configuration which expected to have lower chemical shifts [39Bruno, G; Costantino, L; Curinga, C; Maccari, R; Monforte, F; Nicolo`, F; Ottana`, R; Vigorita, M Synthesis and aldose reductase inhibitory activity of 5-arylidene-2,4-thiazolidinediones Bioorg. Med. Chem, 2002, 10, 1077-1084., 40Vicini, P; Geronikaki, A; Anastasia, K; Incertia, M; Zania, F Synthesis and antimicrobial activity of novel 2-thiazolylimino-5-arylidene-4-thiazolidinones Bioorg. Med. Chem, 2006, 14, 3859-3864.
[http://dx.doi.org/10.1016/j.bmc.2006.01.043] ]. All the newly synthesized compounds were characterized by physical analytical and spectral data and found to be in agreement with predicted values.
Antimicrobial screening
The antibacterial activity of the synthesized compounds was determined by using the cup-diffusion technique [35Wayne, P Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed., Approved standard M7- A5. National Committee for Clinical Laboratory Standards, vol. 20, 2000.] against Staphylococcus aureus and Bacillus subtilis as examples for Gram-positive bacteria, Escherichia coli and Pseudomonas aeruginosa as examples for Gram-negative bacteria and Candida albicans and Candida tropicalis as example for fungi. The antibacterial assay was carried out using NCCLS (National Committee for Clinical Laboratory Standards) broth dilution method. Compounds that exhibited significant inhibition zones were selected for determination of the minimum inhibitory concentrations (MICs). The minimum inhibitory concentrations (MICs) were determined using 96-well microtitre plates [36Pelczar, M J; Chan, E C S; Krieg, N R Microbiology Concepts and Applications, 6th ed; McGraw-Hill Inc: New York, 1999. , 37Collee, J G; Fraser, A G; Marmion, B P; Simmons, A Mackie and McCartney In: Practical medical microbiology, 14th ed; Churchill Livingstone: London, 1996. ].
The results were compared with Ampicillin and Gentamicin for bacteria and Clotrimazole for fungi. The data showed no antifungal activity towards Candida albicans and Candida tropicalis. Whereas, the synthesized compounds exhibited activity against Bacillus subtilis (7a=Ampicillin > 4a=7d) more than Staphylococcus aureus (Ampicillin > 7d > 7a > 4a) as Gram-positive bacteria and weak antibacterial activity was observed against Gram-negative bacteria.
Anti-inflammatory Screening
The anti-inflammatory activity of the synthesized compounds was carried out using carrageenan-induced rat paw edema model [38Winter, C A; Risley, E A; Nuss, G W Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs Proc. Soc. Exp. Biol. Med, 1962, 111, 544-547.
[http://dx.doi.org/10.3181/00379727-111-27849] ]. The results revealed that compounds of thiazolidin-2,4-dione series (7a-7d) exhibited greater anti-inflammatory activity than the 2-iminothiazolidin-4-one series (4a-4d). The best anti-inflammatory activity was observed with compound 7b. The anti-inflammatory activities of other thiazolidin-2,4-dione derivatives were found in the following order (7c>7a>7d). For compounds of iminothiazolidin-4-one series (4a-4d), their anti-inflammatory activities were found to be relatively low and were in the following order (4a>4b>4c>4d).
CONCLUSION
As a final conclusion of this research, new derivatives of both 2-Iminothiazolidin-4-one and thiazolidin-2,4-dione derivatives were synthesized via effective and simple chemical pathways. The target compounds were screened for both antimicrobial and anti-inflammatory activity. Screening results revealed that the anti-inflammatory activity was superior to that of antimicrobial activity. Few compounds exhibited good anti-Gram-positive bacteria and week activity on Gram-negative bacteria. The tested compounds showed no antifungal activity. The thiazolidin-2,4-diones (7a-7d) were found to possess more anti-inflammatory potential than the 2-iminothiazolidin-4-ones (4a-4d). The best anti-inflammatory activity was observed in compound 7b.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors are very grateful for Dr. Abdelbasset A. Farahat, Department of Pharmaceutical Organic Chemistry, Faculty of Pharmacy, Mansoura University for carrying out the NMR spectroscopy at Georgia State University, Atlanta, Georgia USA.
REFERENCES
| [1] | Jain, A K; Vaidya, A; Ravichandran, V; Kashaw, S K; Agrawal, R K Recent developments and biological activities of thiazolidinone derivatives: A review Bioorg. Med. Chem, 2012, 20, 3378-3395. |
| [2] | Abhinit, M; Ghodke, M; Pratima, N A Exploring potential of 4-thiazolidinone: a brief review Int. J. Pharm. Pharm. Sci, 2009, 1, 47-64. |
| [3] | Raza, S; Srivastava, S P; Srivastava, D S; Srivastava, A K; Haq, W; Katti, S B Thiazolidin-4-one and thiazinan-4-one derivatives analogous to rosiglitazone as potential antihyperglycemic and antidyslipidemic agents Eur. J. Med. Chem, 2013, 63, 611-620. |
| [4] | Lee, H W; Kim, B Y; Ahn, J B; Kang, S W; Lee, J H; Shin, J S; Ahn, S K; Lee, S J; Yoon, S S Molecular design, synthesis, and hypoglycemic and hypolipidemic activities of novel pyrimidine derivatives having thiazolidinedione Eur. J. Med. Chem, 2005, 40, 862-874. [http://dx.doi.org/10.1016/j.ejmech.2005.03.019] |
| [5] | Kunzler, A; Neuenfeldt, P D; das Neves, A M; Pereira, C M P; Marques, G H; Nascente, P S; Fernandes, M H V; Hübner, S O; Cunico, W Synthesis, antifungal and cytotoxic activities of 2-aryl-3-((piperidin-1-yl)ethyl)thiazolidinones Eur. J. Med. Chem, 2013, 64, 74-80. |
| [6] | Bonde, C G; Gaikwad, N J Synthesis and preliminary evaluation
of some pyrazine containing thiazolines and thiazolidinones as antimicrobial
agents Bioorg. Med. Chem, 2004, 12, 2151-2161. [http://dx.doi.org/10.1016/j.bmc.2004.02.024] |
| [7] | Chawla, P; Singh, R; Saraf, S K Syntheses and evaluation of 2,5-disubstituted 4-thiazolidinone analogues as antimicrobial agents Med. Chem. Res, 2012, 21, 2064-2071. [http://dx.doi.org/10.1007/s00044-011-9730-1] |
| [8] | Ronad, P M; Noolvi, M N; Sapkal, S; Dharbhamulla, S; Maddi, V S Synthesis and antimicrobial activity of 7-(2-substituted phenylthiazolidinyl)-benzopyran-2-one derivatives Eur. J. Med. Chem, 2010, 45, 85-89. [http://dx.doi.org/10.1016/j.ejmech.2009.09.028] |
| [9] | Sattigeri, V J; Son, A; Singhal, S; Khan, S; Pandya, M; Bhateja, P; Mathur, T; Rattan, A; Khanna, J M; Mehta, A Synthesis and antimicrobial activity of novel thiazolidinones ARKIVOC, 2005, ii, 46-59. |
| [10] | Omar, K; Geronikaki, A; Zoumpoulakis, P; Camoutsis, C; Soković, M; Ćirić, A; Glamočlija, J Novel 4-thiazolidinone derivatives as potential antifungal and antibacterial drugs Bioorg. Med. Chem, 2010, 18, 426-432. |
| [11] | Mori, M; Takagi, M; Noritake, C; Kagabu, S 2,4-Dioxo-1,3-thiazolidine derivatives as a lead for new fungicides J. Pestic. Sci, 2008, 33, 357-363. [http://dx.doi.org/10.1584/jpestics.G08-15] |
| [12] | Vazzana, I; Terranova, E; Mattioli, F; Sparatore, F Aromatic Schiff bases and 2,3-disubstituted-1,3-thiazolidin-4-one derivatives as antiinflammatory agents ARKIVOC, 2004, v, 364-374. [http://dx.doi.org/10.3998/ark.5550190.0005.531] |
| [13] | Prabhakar, C; Madhusudhan, G; Sahadev, K; Maheedhara, R C; Sarma, M R; Om Reddy, G; Chakrabarti, R; Seshagiri, C R; Kumar, K D; Rajagopalan, R Synthesis and biological activity of novel thiazolidinediones Bioorg. Med. Chem. Lett, 1998, 8, 2725-2730. |
| [14] | Amla, A; Youssef, M; White, S; Erika, B; Ibrahim, M Synthesis and biological evaluation of novel pyrazolyl-2,4-TZD as anti-inflammatory and neuro protective agents Bioorg. Med. Chem, 2010, 18, 2019-2028. [http://dx.doi.org/10.1016/j.bmc.2010.01.021] |
| [15] | Ceriello, A Thiazolidinediones as anti-inflammatory and anti-atherogenic agents Diabetes Metab. Res. Rev, 2008, 24, 14-26. [http://dx.doi.org/10.1002/dmrr.790] |
| [16] | Ialenti, A; Grassia, G; Di Meglio, P; Maffia, P; Di Rosa, M; Ianaro, A Mechanism of the anti-inflammatory effect of thiazolidinediones: relationship with the glucocorticoid pathway Mol. Pharmacol, 2005, 67, 1620-1628. |
| [17] | Goel, B; Ram, T; Tyagi, R; Bansal, E; Kumar, A; Mukherjee, D; Sinha, J N 2-Substituted-3-(4-bromo-2-carboxyphenyl)-5-methyl-4-thiazolidinones as potential anti-inflammatory agents Eur. J. Med. Chem, 1999, 34, 265-269. [http://dx.doi.org/10.1016/S0223-5234(99)80060-9] |
| [18] | Geronikaki, A A; Lagunin, A A; Hadjipablou-Litina, D I; Eleftheriou, E T; Filimonov, D A; Poroikov, VV; Alam, I; Saxena, A A Computer-aided discovery of anti-inflammatory thiazolidinones with dual cyclooxygenase/lipoxygenase inhibition J. Med. Chem, 2008, 51, 1601-1609. |
| [19] | Wu, Y; Karna, S; Choi, CH; Tong, M; Tai, H H; Na, D H; Jang, C H; Cho, H Synthesis and biological evaluation of novel thiazolidinedione analogues as 15-hydroxyprostaglandin dehydrogenase inhibitors J. Med. Chem, 2011, 54, 5260-5264. [http://dx.doi.org/10.1021/jm200390u] |
| [20] | Ergenc, N; Capan, G Synthesis and anticonvulsant activity of new 4-thiazolidone and 4-thiazoline derivatives IL Farmaco, 1994, 49, 133-135. |
| [21] | Dwivedi, C; Gupta, T K; Parmar, S S Substituted thiazolidones as anticonvulsants J. Med. Chem, 1972, 15, 553-554. [http://dx.doi.org/10.1021/jm00275a031] |
| [22] | Hafez, H N; El-Gazzar, A R Synthesis and antitumor activity of substituted triazolo[4,3-a]pyrimidin-6-sulfonamide with an incorporated thiazolidinone moiety Bioorg. Med. Chem. Lett, 2009, 19, 4143-4147. [http://dx.doi.org/10.1016/j.bmcl.2009.05.126] |
| [23] | Reddy, K A; Lohray, B B; Bhushan, V; Reddy, A S; Kishore, P H; Rao, V V; Saibaba, V; Bajji, A C; Rajesh, B M; Reddy, K V; Chakrabarti, R; Rajagopalan, R Novel euglycemic and hypolipidemic agents: Part-2 antioxidant moiety as structural motif Bioorg. Med. Chem. Lett, 1998, 8, 999-1002. [http://dx.doi.org/10.1016/S0960-894X(98)00159-0] |
| [24] | Vicini, P; Zani, F; Cossini, P; Doytchinova, I Hydrazones of 1,2-benzisothiazole hydrazides: synthesis, antimicrobial activity and QSAR investigations Eur. J. Med. Chem, 2002, 37, 553-564. [http://dx.doi.org/10.1016/S0223-5234(02)01378-8] |
| [25] | Ottana`, R; Maccari, R; Barreca, M L; Bruno, G; Rotondo, A; Rossi, A; Chiricosta, G; Paola, R D; Sautebin, L; Cuzzocread, S; Vigorita, M G 5-Arylidene-2-imino-4-thiazolidinones: Design and synthesis of novel anti-inflammatory agents Bioorg. Med. Chem, 2005, 13, 4243-4252. |
| [26] | Chavan, A A; Pai, N R Synthesis and antimicrobial screening of 5-arylidene-2-imino-4-thiazolidinones ARKIVOK, 2007, xvi, 148-155. [http://dx.doi.org/10.3998/ark.5550190.0008.g16] |
| [27] | Sharma, P K; Chandak, N; Kumar, P; Sharma, C; Aneja, K R Synthesis and biological evaluation of some 4-functionalized-pyrazoles as antimicrobial agents Eur. J. Med. Chem, 2011, 46, 1425-1432. [http://dx.doi.org/10.1016/j.ejmech.2011.01.060] |
| [28] | Bekhit, A A; Fahmy, H T Y; Rostom, S A F; Bekhit, A E A Synthesis and biological evaluation of some thiazolylpyrazole derivatives as dual anti-inflammatory antimicrobial agents Eur. J. Med. Chem, 2010, 45, 6027-6038. [http://dx.doi.org/10.1016/j.ejmech.2010.10.001] |
| [29] | Apostolidis, I; Liaras, K; Geronikaki, A; Hadjipavlou-Litina, D; Gavalas, A; Soković, M; Glamočlija, J; Ćirić, A Synthesis and biological evaluation of some 5-arylidene-2-(1,3-thiazol-2-ylimino)-1,3-thiazolidin-4-ones as dual anti-inflammatory/antimicrobial agents Bioorg. Med. Chem, 2013, 21, 532-539. |
| [30] | Alsughayer, A; Elassar, A A; Mustafa, S; Al Sagheer, F Synthesis, structure analysis and antibacterial activity of new potent sulfonamide derivatives J. Biomat. Nanobiotech, 2011, 2, 144-149. [http://dx.doi.org/10.4236/jbnb.2011.22018] |
| [31] | Sohda, T; Mizuno, K; Imamiya, E; Sugiyama, Y; Fujita, T; Kawamasu, Y Studies on Antidiabetic Agents. II. Synthesis of 5-[4-(1-Methylcyclohexylmethoxy)-benzyl]thiazolidine-2,4-dione (ADD-3878) and its derivatives Chem. Pharm. Bull, 1982, 30, 3580-3600. |
| [32] | Ha, YM; Park, YJ; Kim, J; Park, D; Park, J Y; Lee, H J; Lee, J Y; Moon, H R; Chung, H Y Design and synthesis of 5-(substituted benzylidene)thiazolidine-2,4-dione derivatives as novel tyrosinase inhibitors Eur. J. Med. Chem, 2012, 49, 245-252. |
| [33] | Jacobs, W A; Heidelberger, M J On amides, uramino compounds, and ureides containing an aromatic nucleus J. Am. Chem. Soc, 1917, 39, 2418-2443. [http://dx.doi.org/10.1021/ja02256a021] |
| [34] | Taniyama, H; Takemura, S Studies on thiazole derivatives. I. Synthesis of 4-thiazolidones Yakugaku Zasshi, 1953, 73, 164-165. |
| [35] | Wayne, P Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed., Approved standard M7- A5. National Committee for Clinical Laboratory Standards, vol. 20, 2000. |
| [36] | Pelczar, M J; Chan, E C S; Krieg, N R Microbiology Concepts and Applications, 6th ed; McGraw-Hill Inc: New York, 1999. |
| [37] | Collee, J G; Fraser, A G; Marmion, B P; Simmons, A Mackie and McCartney In: Practical medical microbiology, 14th ed; Churchill Livingstone: London, 1996. |
| [38] | Winter, C A; Risley, E A; Nuss, G W Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs Proc. Soc. Exp. Biol. Med, 1962, 111, 544-547. [http://dx.doi.org/10.3181/00379727-111-27849] |
| [39] | Bruno, G; Costantino, L; Curinga, C; Maccari, R; Monforte, F; Nicolo`, F; Ottana`, R; Vigorita, M Synthesis and aldose reductase inhibitory activity of 5-arylidene-2,4-thiazolidinediones Bioorg. Med. Chem, 2002, 10, 1077-1084. |
| [40] | Vicini, P; Geronikaki, A; Anastasia, K; Incertia, M; Zania, F Synthesis and antimicrobial activity of novel 2-thiazolylimino-5-arylidene-4-thiazolidinones Bioorg. Med. Chem, 2006, 14, 3859-3864. [http://dx.doi.org/10.1016/j.bmc.2006.01.043] |