- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Cell Development & Biology Journal
(Discontinued)
ISSN: 1874-0855 ― Volume 3, 2011
LET-756/FGF is Implicated in the Control of C. elegans Body Size
Cornel Popovici1, 2, Sylvain Hiver1, Daniel Birnbaum1, Régine Roubin*, 1
Abstract
LET-756 is one of the two fibroblast growth factors (FGF) of Caenorhabditis elegans. We have previously shown that worms homozygote for the let-756 null allele (s2887) die at larval stage and that homozygotes for the partial loss-of-function allele (s2613) are viable but shorter than wild-type animals. We now show that expression of let-756 in any region of the s2887 worm can rescue the lethal phenotype but with variations in body size depending on the site of expression, harmonious growth being achieved by let-756 expression in body wall muscles. Local action of let-756 was also observed in mosaic animals homozygotes for the s2613 allele. Our results support a paracrine activity for LET-756 exerting its effect locally on body size whether by acting as an actual growth factor or by acting on EGL-15/FGFR expressing hypodermal cells thereby regulating fluid balance.
Article Information
Identifiers and Pagination:
Year: 2008Volume: 1
First Page: 24
Last Page: 32
Publisher Id: TOCBJ-1-24
DOI: 10.2174/1874085500801010024
Article History:
Received Date: 13/8/2008Revision Received Date: 15/9/2008
Acceptance Date: 16/9/2008
Electronic publication date: 9/10/2008
Collection year: 2008
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Inserm UMR891, Centre de Recherche en Cancérologie de Marseille, Institut Paoli-Calmettes, Université de la Méditerranée, 13009, Marseille, France; E-mail: regine.roubin@inserm.fr
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 13-8-2008 |
Original Manuscript | LET-756/FGF is Implicated in the Control of C. elegans Body Size | |
INTRODUCTION
In vertebrates, Fibroblast Growth Factors (FGFs) are involved in various developmental and pathological processes (for review see [1]), usually in combination with other regulatory growth factors, such as members of WNT and TGF superfamilies. In humans, 22 FGFs are distributed in seven families [2]. FGFs activate transmembrane FGF tyrosine kinase receptors (FGFR). Activated FGFRs dimerize, are phosphorylated, and signal through cytoplasmic proteins. Coreceptors, such as heparan sulphate proteoglycans, are required for proper membrane signaling. A special family of FGFs, the fibroblast homologous factors (FHF), has different functions and signaling pathways [3]. Some FGFs may signal through a direct nuclear pathway since FGFs or both FGFs and FGFRs have been identified in the nucleus (reviewed in [4]).
Only a few FGFs have been found in protostomian species whose sequences are available. In the worm Caenorhabditis elegans, two FGFs, EGL-17 and LET-756, and one FGFR, EGL-15, have been described [5-7]. An EGL-17 mutant has a defect in migration of sex muscles, and consequently in vulva function and egg laying [8, 9]. LET-756 is essential for worm development [10]. It regulates axonal growth [11], fluid homeostasis [12], muscle protein degradation [13] and muscle membrane extension [14].
In C. elegans, three different pathways determine body length: a TGFβ pathway, involving dbl-1, sma-2, sma-3, sma-4, lon-1 and lon-2 genes, a spectrin pathway involving sma-1, spc-1 and unc-70 and a calcineurin pathway involving sensory neurons and muscle cells expressing tax-6 and cnb-1 [15-19]. An underlying ectodermal cell layer called the hypodermis makes cuticle, an exoskeleton of collagenous extracellular matrix (ECM). The hypodermis surrounds the body of the animal [reviewed in 20]. Proteins implicated in body length act on or are expressed in hypodermal cells. Thus, the hypodermis is the primary site where growth is regulated.
LET-756/FGF partial loss-of-function mutants are of the same size as wild-type (wt) animals at hatching, but grow more slowly during larval stages and are about half the length of, and are thinner than wt worms in adulthood [10]. Reduction in body size could be due to reduced cell number, reduced cell size or fluid loss. Because no difference in the number of nuclei in wt and let-756 (s2613) mutants has been found [10], the small body size phenotype could be due to a reduction in size of some or all cells of the animal or to body fluid loss.
To document the effect of let-756 on body size we performed rescue experiments of both the partial loss-of-function allele (s2613) and the null allele (s2887), followed by a morphometric analysis of the body size of the rescued animals. To determine which site of expression was important for rescue, let-756 was expressed in various areas of the body.
MATERIALS AND METHODOLOGY
Nematode Culture and Transformation Rescue Experiments
C. elegans Bristol (N2) strain nematodes were cultured using standard techniques [21]. let-756 rescuing activity was assayed by injecting tester DNA at 50 ng per µl into gravid s2887 strain hermaphrodites, establishing transformed lines and scoring the Dpy progeny for a viable phenotype. The s2887 allele used in these experiments was generated on a dpy-17(e164) unc-32(e189) chromosome after UV irradiation [22]; the sDp3, a portion of chromosome III, balances the lethal phenotype of the s2887 strain. The rescue activity was tested for the presence in the progeny of live Dpy animals. Dpy animals were genotyped for the presence of the inversion in the let-756 locus characteristic of the s2887 strain by PCR on single worm, as described in [23]. The strains dpy-17(e164) unc-32 (e189) and unc-32 (e189) used as control, were obtained from the Caenorhabditis Genetics Center (CGC).
The hypomorph FF628 let-756(s2613) unc-32(e189) strain was isolated by D. Thierry-Mieg from BC4253 and outcrossed an additional 7 times. Homozygous animals are viable and fertile, but slow growing, transparent and small. These animals were coinjected with Plet-756::let-756 and the pPD93.97 plasmid which contains Pmyo3::GFP. Fluorescent cells contain arrays bearing both plasmids and mosaic animals were analyzed.
Fusion of Tissue Specific Promoters and let-756::gfp Coding Region
The promoters used in muscle-specific expression of let-756::gfp were myo-2 (pharyngeal muscles), myo-3 (body muscles) and egl-17 (sex muscles) [24, 8]. The constructs driving the coding region of let-756::gfp and let-756R318::gfp under the control of Plet-756 promoter have already been reported [25]. The Pmyo-3::let-756::gfp expression construct was made by ligating the PCR product containing the let-756 cDNA in the XbaI-BamHI digested pPD93.97. The XbaI-EcoRI fragment containing the let-756::gfp fusion was then excised and inserted in the NheI-BglII digested pPD96.48 vector to obtain Pmyo-2::let-756::gfp expression construct. The full length let-756 genomic DNA under the control of egl-17 promoter (NH#469) was a gift from M. Stern (Yale, USA).
Microscopy and Statistical Analysis
The method used for immunofluorescence experiment was essentially as described by Ruvkun and Giusto [26]. Animals were fixed in a mixture of 1% paraformaldehyde, 20% methanol, permeabilized in 1% triton. Fixed animals were then incubated overnight with a 1:400 dilution of anti-GFP antiserum (Roche), washed extensively and then incubated for another two hours in a 1:500 dilution of the anti- mouse antibody conjugated to Texas Red (Molecular Probes). After extensive washing, the animals were mounted in DAPI-containing glycerol solution. In some experiments, DiI (Molecular Probes) dye filling was performed on living preparations of worms as previously described [27] to identify chemosensory neurons [28].
To analyze phenotypes, worms were collected on agar pads, anesthetized with levamisol and observed on a Zeiss LSM 510 laser scanning microscope equipped with Normasky optics. The number of s2887 transformed strains analyzed was: 2 for the Plet-756:: let-756::gfp construct, 3 for Plet-756:: let-756R318::gfp, 3 for P myo-3::let-756::gfp, 4 for Pmyo-2::let-756::gfp and 2 for Pegl-17::let-756. Three s2613 strains transformed by Plet-756::let-756::gfp were analyzed. In the wild type context, 3 5 [rol] strains were compared to 2 strains transformed with Plet-756::let-756::gfp and two strains transformed with Plet-756:: let-756R318::gfp.
To assess body size in transgenic animals, cultures were synchronized by bleaching gravid animals. Eggs were allowed to grow for 90 hours in the adult stage. Selected plates containing adult rescued [dpy] animals were washed off in M9 medium and fixed in a mixture of 1% paraformaldehyde, 20% methanol. Body sizes (length and width at the vulva) were measured on 100 individual animals using LSM 510 software. Non-parametric Mann-Whitney u test was used. Differences were considered as significant when p values were < 0.01.
RESULTS
LET-756 Expression
To determine where let-756 is required to insure worm viability, we examined the cells that express a GFP reporter from ~ 4 kb of promoter and enhancer sequences of let-756 (Plet-756::gfp). Animals carrying this transgene express GFP in many cells throughout development and in the adult, as reported by us and others [7, 11, 14].
The major site of let-756 expression was found in descendant cells of mesodermic precursors (muscle cells). However, some cephalic neurons, CAN cells, and the two glandular cells G1 and G2 (Fig. 1A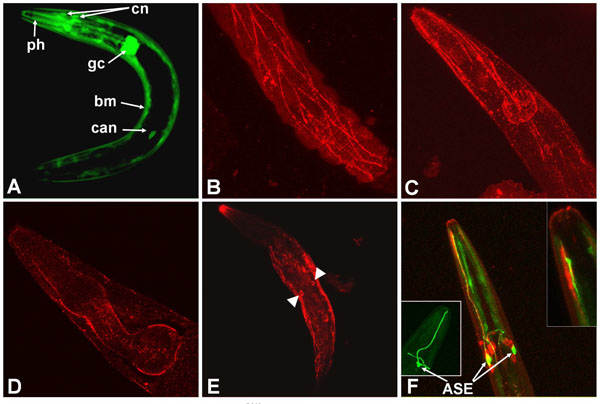 ) were also positive.
) were also positive.
Because LET-756 is secreted [23], we expected to find the FGF at some distance from its site of synthesis. We compared the sites of expression to those of LET-756 action as revealed by the localization of a LET-756::GFP fusion. We used a construct where identical let-756 upstream sequence drives the LET-756::GFP fusion protein. We found accumulation of LET-756::GFP mainly in body wall muscle cells and incidentally in vulva, sphincter and pharyngeal muscle cells. However, under microscope observation only few animals were positive for LET-756::GFP despite the fact that they were perfectly rescued; in addition, the fluorescence was often lost with time in rescued strains although the rescuing ability was not.
To correlate protein level expression with rescue capacity we performed immunofluorescence staining of the rescued animals with anti-GFP antibody. Immunodetection of GFP is more sensitive than the observation of its direct fluorescence when exposed to UV light and thus more suitable to detect small amounts of protein. Immunostaining was seen in all rescued individuals, even in the absence of the GFP direct fluorescence, indicating that only animals containing a small number of arrays were able to survive and that these arrays did not contain enough copies of GFP to allow visualization by direct fluorescence.
After GFP immunostaining, animals displayed accumulation of fluorescence in a punctuate pattern. It was not possible to discriminate between a localization of LET-756 at the cell membrane on the cytoplasm side or, more likely, extracellularly on the basement membrane of body wall muscle cells (Fig. 1B ,C
,C ) and around the pharynx (Fig. 1D
) and around the pharynx (Fig. 1D ), in regions where collagen [29] and UNC-52/perlecan [30] accumulate.
), in regions where collagen [29] and UNC-52/perlecan [30] accumulate.
The fusion was observed in the nucleus and not in the nucleolus of CAN cells (Fig. 1E ), in agreement with the results obtained with Cos-1 transfected mammalian cells [25]. A construct that mimicked the partial loss-of-function allele, which we have previously denominated LET-756R318, fused to GFP and under the control of the same upstream sequence, was expressed in muscle cells. Surprisingly, it was also expressed in some neurons exhibiting peculiar endings unable to be filled by the DiI lipophilic dye (Fig. 1F
), in agreement with the results obtained with Cos-1 transfected mammalian cells [25]. A construct that mimicked the partial loss-of-function allele, which we have previously denominated LET-756R318, fused to GFP and under the control of the same upstream sequence, was expressed in muscle cells. Surprisingly, it was also expressed in some neurons exhibiting peculiar endings unable to be filled by the DiI lipophilic dye (Fig. 1F ), which we tentatively identified as the chemosensorial ASE neurons. We never observed this type of fluorescent cells when using the let-756 promoter driving GFP. One possibility is that this staining reflects the specific translocation of the mutated protein in this cell type, but we do not have any definite explanation for this observation. Unlike cells with wt FGF, the nucleolus was also stained in these cells.
), which we tentatively identified as the chemosensorial ASE neurons. We never observed this type of fluorescent cells when using the let-756 promoter driving GFP. One possibility is that this staining reflects the specific translocation of the mutated protein in this cell type, but we do not have any definite explanation for this observation. Unlike cells with wt FGF, the nucleolus was also stained in these cells.
Lethality is Rescued by Tissue-Specific Expression of LET-756
let-756 is expressed mainly in various muscle cells. To determine which of these locations are essential for viability, we created transgenic animals expressing let-756 in various muscles. Constructs were made with tissue-specific promoters and the let-756::gfp C-terminal fusion. For body wall muscle expression we used Pmyo-3::let-756 [24] and for pharyngeal expression, we used Pmyo-2::let-756 [24]. For sex myoblast expression, we used Pegl-17::let-756 [9], which drives expression specifically in primary fate vulval precursors and their descendants. These constructs were introduced in animals carrying the let-756(s2887) null allele [dpy->9)] and animals were examined for both rescue activity and phenotype. In addition, we compared the rescuing activity of the wt LET-756 molecule with its truncated form LET-756R318, which mimics the partial loss-of-function allele (s2613).
All types of tested ectopic muscle expression rescued the lethal phenotype: 7, 6 and 3 strains were obtained using myo-3, myo-2 and egl-17 promoters respectively, indicating that muscle expression is sufficient to insure viability.
Worm Body Growth Depends on Spatial Expression of LET-756
Variations in body length and proportions were observed in the various rescued animals described above. To identify the action of spatially-restricted gene expression on body length and proportions we analyzed transgenic animals with wt let-756 expression driven by both endogenous and heterologous promoters in the null allele (s2887) background. Three types of spatial expression patterns were studied: two spatially-restricted expression patterns (anterior and central regions of the worm body, corresponding to the expression of the transgene under the control of the heterologous promoters Pmyo-2 and Pegl-17, respectively) [24, 9] and a widespread expression pattern corresponding to the expression of the transgene under the control of the endogenous and Pmyo-3 promoters [24].
Body sizes of animals were measured - without assessing the size of individual cells - in these different conditions. Individual total body length, mouth-to-vulva length, and width at the vulva are shown in Table 1.
Expression of wt let-756 from the myo-3 promoter produced animals with the same characteristics as those rescued by using the let-756 promoter (Fig. 2B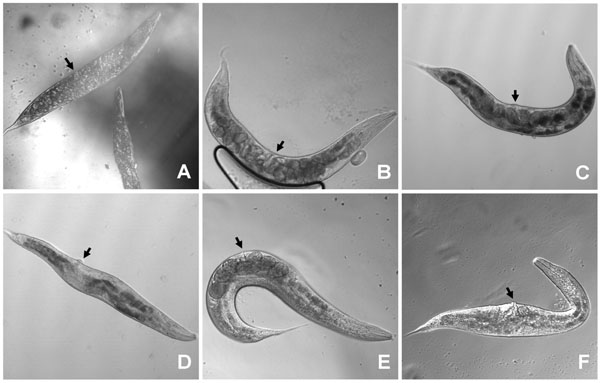 ,2E
,2E ). MYO-3 is expressed in body wall muscles, which are distributed all along the body from head to tail. Therefore, LET-756 expression in body wall muscles is sufficient for full rescue of the lethal phenotype and insures the same body length development as expression driven by the proper promoter. The respective proportions of anterior and posterior body parts in strains rescued with Plet-756and Pmyo-3 promoters being similar (Fig. 3
). MYO-3 is expressed in body wall muscles, which are distributed all along the body from head to tail. Therefore, LET-756 expression in body wall muscles is sufficient for full rescue of the lethal phenotype and insures the same body length development as expression driven by the proper promoter. The respective proportions of anterior and posterior body parts in strains rescued with Plet-756and Pmyo-3 promoters being similar (Fig. 3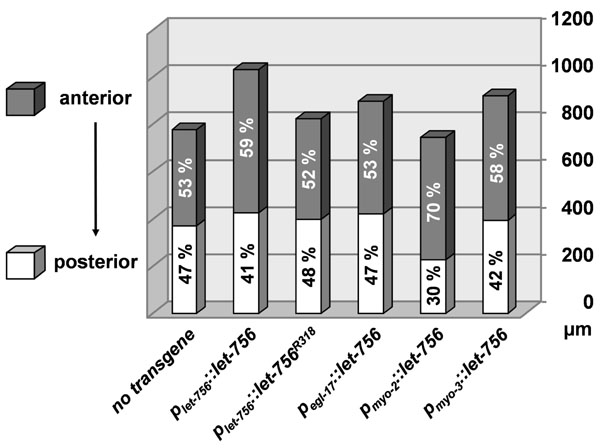 ), the contribution of pharyngeal LET-756 expression seems to be minor.
), the contribution of pharyngeal LET-756 expression seems to be minor.
 |
Fig. (2) Worms transformed with let-756 under the control of tissue specific promoters were phenotypically different. Worms transformed by let-756 (B) or let-756R318 (C) under the control of Plet-756 promoter are not different from the control [Dpy Unc] strain (A) although the former are bigger (see Fig. (3 |
 |
Fig. (3) Morphometric analysis. Anterior (shaded area) and posterior (unshaded) sizes of the different strains are represented (in µm) as well as their relative contribution in the total body length. |
Expression of LET-756 from the Pmyo-2 promoter, which drives expression exclusively in the pharynx musculature, led to shorter animals than the one described above. In addition, they exhibited a misplaced vulva due to a better development of the anterior (defined as the length from mouth to vulva) than the posterior part of the body (Fig. 2D ). These animals exhibited a ratio “anterior length over total” larger than control individuals or individuals with LET-756 or LET-756R318 expression driven by Plet-756 and Pmyo-3 (Table 1).
). These animals exhibited a ratio “anterior length over total” larger than control individuals or individuals with LET-756 or LET-756R318 expression driven by Plet-756 and Pmyo-3 (Table 1).
Expression of LET-756 from the egl-17 promoter led to animals with larger width size than control animals (Fig. 2F ). EGL-17 is expressed in sex muscles [9]. It is thus coherent that LET-756 expression driven by the egl-17 promoter in the middle of the animal increased locally the size of the animals. In addition, the posterior length was also increased compared to control strain and animals rescued with the let-756R318 transgene. Sex muscles arise from a pair of bilaterally symmetrical sex myoblasts; these cells are born in the posterior body. During larval development of the transgenic animals, enough LET-756 vehiculated by migrating myoblasts may allow the growth of the posterior part of the animals.
). EGL-17 is expressed in sex muscles [9]. It is thus coherent that LET-756 expression driven by the egl-17 promoter in the middle of the animal increased locally the size of the animals. In addition, the posterior length was also increased compared to control strain and animals rescued with the let-756R318 transgene. Sex muscles arise from a pair of bilaterally symmetrical sex myoblasts; these cells are born in the posterior body. During larval development of the transgenic animals, enough LET-756 vehiculated by migrating myoblasts may allow the growth of the posterior part of the animals.
Reduced Body Size Results from let-756R318 Transgene Expression
We have previously reported that no major morphological and/or behavior differences was observed between lines rescued with Plet-756::let-756 and Plet-756::let-756R318 constructs [25], which mimic wt and partial-loss-of-function alleles, respectively. However, in light of our results on body size we analyzed the length and width of these transgenic animals.
As compared to [dpy-17(e164) unc-32(e189)] animals, which have only two copies of the wt let-756 gene, animals from the s2887 strain (null allele) rescued by let-756 expressed under the control of its own promoter had enlarged body length mostly due to a better development of the anterior part of the animal (Table 1, Fig. 2A ,B
,B ). In contrast, the body length of animals rescued by let-756R318 was smaller than the length of those rescued by the wt molecule, and was in the range of the body length of the control (dpy-17 (e164) unc-32(e189)) animals (Figs. 2C
). In contrast, the body length of animals rescued by let-756R318 was smaller than the length of those rescued by the wt molecule, and was in the range of the body length of the control (dpy-17 (e164) unc-32(e189)) animals (Figs. 2C ,3
,3 ). As already reported [29], we found a four-fold increase in the number of transgene copies in let-756R318 strains compared to strains rescued with the wt let-756. This increase in array copy number could be explained by different activities of the proteins encoded by transgenes on the tyrosine kinase FGFR/EGL-15, since both FGFs are secreted (23) or by the different subcellular localizations of the two molecules in the expressing cells [25].
). As already reported [29], we found a four-fold increase in the number of transgene copies in let-756R318 strains compared to strains rescued with the wt let-756. This increase in array copy number could be explained by different activities of the proteins encoded by transgenes on the tyrosine kinase FGFR/EGL-15, since both FGFs are secreted (23) or by the different subcellular localizations of the two molecules in the expressing cells [25].
Thus, in all circumstances, LET-756 increased the animal body size in areas where it was expressed and body muscle cell expression was sufficient for ensuring normal body length. Quantification indicated that, as compared to [dpy-17(e164) unc-3(e189)] animals, the total length of all transgenic animals (except for Pmyo-2::let-756) was significantly increased (p < 0.01) in a hierarchy Plet-756::let-756 > Pmyo-3::let-756 > Plet-756::let756R318 = Pegl-17::let-756> no transgene > Pmyo-2::let-756. In contrast, the anterior part of animals transformed with Pmyo-2::let-756 was significantly (p <10-5) larger than untransformed worms or worms transformed with Pegl-17::let-756 or Plet-756::let756R318 transgene. It was not different from worms transformed with Pmyo-3::let-756 and smaller than those transformed with Plet-756::let-756. The posterior part of Pmyo-2::let-756 was shorter than all the other transgenic or wild-type strains (p <10-7). Finally, the width of Pmyo-2::let-756 and Pegl-17::let-756 transgenic strains was increased (p <10-4) as compared to all other animals (Fig. 3 ).
).
let-756 Effect on Body Length Does Not Depend on Genetic Background
Some genes coding for cuticle collagens modify worm body length [20]. We were thus concerned by a possible effect of dpy-17 (20), which was used as a balancer in the establishement of homozygote strain for let-756 loss-of-function allele, and rol-6 [31], a largely used coinjection marker of transformation, both encoding cuticle collagens.
Due to early lethality of the let-756 loss-of-function allele, we could not compare the size of rescued vs unrescued animals. We thus compared the ratio between body length and width in the various rescued strains, as a hallmark of the Dpy phenotype. Dpy mutants are defined as small, fatty worms and thus display a larger width to length size ratio than wt animals. The body dimensions were increased in the rescued strains expressing the wt let-756 transgene under the control of the Plet-756 or Pmyo-3 compared to the dpy-1((e164) unc-3(e189) reference strain, but, importantly, the width to length size ratio was not modified (Table 1). Thus, overexpression of let-756 cannot suppress the Dpy phenotype of the dpy-17 mutant allele.
To eliminate unambiguously any effect of the dpy-17 background on the LET-756-induced body length, we compared the consequence of let-756 transgene expression under the control of its own promoter in the [let-756(s2613) unc-32(e189)] hypomorph as well in wt N2 animals. In the hypomorph, the myo-3 marker was used as an indicator of which area of the body was transformed, and in wt N2 animals, the rol-6 marker was used to identify transformed animals. Expression of LET-756 increased both length and width in the [let-">9)] hypomorph in which the dpy-17 mutation had being outcrossed (Table 2). As compared to the hypomorph (Fig. 4A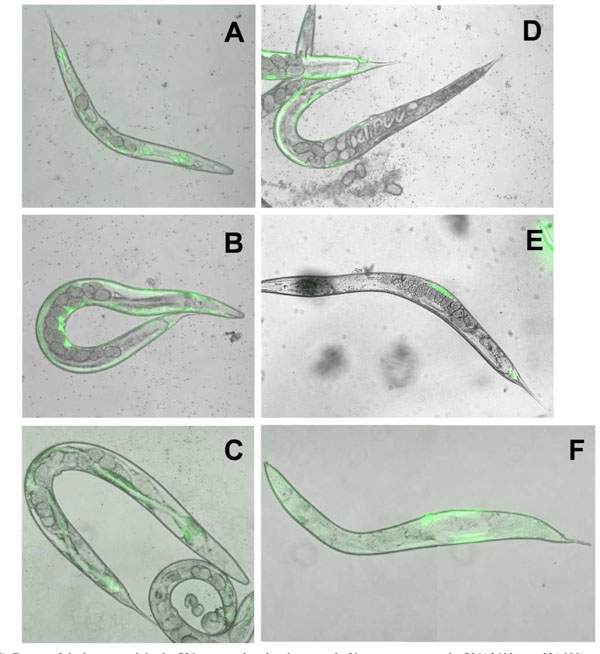 ), the rescued animals appeared larger and developed a normal number of eggs (Fig. 4B
), the rescued animals appeared larger and developed a normal number of eggs (Fig. 4B ). They did not look different from the unc-32 (e189) strain (Fig. 4C
). They did not look different from the unc-32 (e189) strain (Fig. 4C ), which contains two wt let-756 alleles. However, in mosaic animals where LET-756 was expressed only in the anterior part of the animal, the posterior part looked scrawny and thinner than normal (Fig. 4D
), which contains two wt let-756 alleles. However, in mosaic animals where LET-756 was expressed only in the anterior part of the animal, the posterior part looked scrawny and thinner than normal (Fig. 4D ). Reciprocally, animals where FGF expression occurred only in the posterior part of the animals exhibited a thinner anterior part (Fig. 4E
). Reciprocally, animals where FGF expression occurred only in the posterior part of the animals exhibited a thinner anterior part (Fig. 4E ,F
,F ). Thus, the same phenotypes as those described in the worms carrying the loss-of-function allele s2887 were observed in the animals carrying the partial loss-of-function allele s2613, showing that spatial expression of LET-756 increases body size independently of the genetic background.
). Thus, the same phenotypes as those described in the worms carrying the loss-of-function allele s2887 were observed in the animals carrying the partial loss-of-function allele s2613, showing that spatial expression of LET-756 increases body size independently of the genetic background.
Finally, we examined the effect of let-756 over-expression in a wt context. We found that N2 animals injected with with Plet-756::let-756 or Plet-756::let756R318 were not different in size (Table 3) than those injected with Plet-756::gfp although smaller than untransformed worms, due to the presence of rol-6 (su1006) allele, the transformation marker gene. Thus, overexpression of LET-756 or LET-756R318 in a wt context did not result in an overgrowth phenotype. A threshold ligand amount expressed by endogenous alleles of the N2 worms and sufficient to saturate the EGL-15 receptors may explain size conservation.
DISCUSSION
Body size control is an oligogenic/polygenic character that depends on both nutrition-dependent and independent pathways. Studies in Drosophila melanogaster and Caenorhabditis elegans highlighted the insulin pathways as the principal player in controling nutrition-dependent growth rates [32 and 33 for reviews]. In the nematode worm, three nutrition-independent pathways (a TGFβ pathway, a spectrin pathway and a calcineurin pathway) determine body length by acting on the hypodermis, the primary site of growth regulation. The size of hypodermal cells and the composition of cuticle, the exoskeleton surrounding the body of the worm synthesized by hypodermal cells, are the main factors affecting body size.
In this study, we demonstrate that the FGF pathway is implicated in the control of the worm body size. Although let-756 loss-of-function allele (s2887) is lethal, individuals carrying the partial loss-of-function allele (s2613) grow very slowly into small transparent, starved-looking adults. Explanations could be defects in cells that make cuticle or dysfunction of the pharyngeal musculature.
To determine the focus of lethality and to understand the defect in body size we analyzed which let-756-expressing cell was responsible for correct larval development. We focused on the role of different let-756 expressing muscle cells in allowing post-embryonic development rescue and found that expressing let-756 under the control of the myo-3 promoter induced the same harmonious development of the animal as expressing the endogenous protein under the control of the proper promoter. Body length is controled by hypodermal cells, which make cuticle. Hypodermal cells express EGL-15, the FGF receptor. Most probably, LET-756, through paracrine expression in muscle cells, acts on the hypodermal cells, triggers EGL-15 signaling, which regulates fluid balance [12] and cuticle formation. Thus, muscle expression of LET-756 is sufficient to obtain a well developed worm and may be essential for proper development of the nematode C. elegans. It is worth noting that the promoter region of let-756 contains a muscle-specific responsive element also present in the myo-3 but not myo-2 promoter [34].
Another explanation for the impaired growth of the let-756 mutant could be a defect in the pharyngeal musculature, impairing the pumping or grinding of bacteria - the nematode food source - or in pharyngeal gland cells. The function of the latter cells is unknown; their anatomy indicates that they are secretory cells, pouring their content in the lumen of the pharynx. Pharynx and gland cells are two sites were LET-756 is expressed. Driving LET-756 expression in the pharyngeal musculature from the myo-2 promoter confers viability to the null allele but the rescue looked incomplete since only the anterior part of the animal developed correctly. Thus, we can exclude a pharyngeal cell-autonomous action of LET-756 responsible for an either mechanistic or digestive problem of feeding. Interestingly, body width was increased in worms transformed by Pmyo-2::let-756. However, these animals appeared very often [Egl], indicating that the limited available amount of LET-756 at middle body (resulting from the pharynx muscle cells expression) could interfere with EGL-15 activation and not allow proper sex myoblast positioning and/or progeny formation (uterine and vulval muscles). Thus, LET-756 may constitute, alone or in cooperation with other diffusible ligands, the gonad-independent attraction signal for sex myoblast cells described previously [9].
Body length was increased when let-756 was expressed under the control of the egl-17 promoter. This was mainly due to a well-developed posterior part of the animal. EGL-17, the other C. elegans FGF, is expressed in the developing gonad and vulva and acts as the chemoattractant that guides the migration of the sex myoblasts. During sex muscles migration from the posterior body, enough LET-756 vehiculated by migrating myoblasts may allow the growth of the posterior part of the animal. In addition, body width was increased indicating again that LET-756 increases body mass wherever it is produced.
Thus, any spatial rescue results in a local growth of the animal leaving the other part of the animal with a scrawny phenotype. This may result from either abnormal cell growth or from worm lacking sufficient fluid as described by Huang and Stern [12], as observed in the null or partial loss-of-function allele. We suggest that overexpression of LET-756 from a small number of cells - and herein from any cells of mesodermal origin - might be sufficient to regulate fluid balance and trigger EGL-15 activity on hypodermal cells.
Our observations suggest that wherever LET-756 is expressed and acts on its receptor on hypodermal cells, post-embryonic development is insured. The extracellular matrix components are essential players in the regulation of local concentration of growth factors and morphogens. Experimental observations showed that glypicans, cell-anchored heparan sulfate proteoglycans, bind growth factors such as FGF in Drosophila [35] and TGFβ in C. elegans [19], modulating their local concentration and protecting them from proteolytic degradation. We have observed accumulation of LET-756 around the pharynx and in the extracellular space of body muscle cells. Collagens are critical for the assembly and function of the extracellular matrix. It is noteworthy that we found COL-129, the product of a structural collagen gene, interacting with LET-756 in yeast two-hybrid screens [36].
Dixon et al. [14] reported that expression of LET-756 in a single cell is sufficient to rescue the lethality of let-756(s2887) null animals and that the rescued animals have a pronounced bulge near the site of expression. Our results indicate that even if the animals are capable of surviving when only one cell type is expressing LET-756, normal body growth is limited to the area where LET-756 can diffuse.
In summary, our data show that misexpression of LET-756, as in the partial loss-of-function allele, reduces the size of the animal as compared to wild-type. Reciprocally, LET-756 overexpression in the hypomorph or the null allele increases body size. Expression in body muscles is sufficient to allow the development of the null let-756 allele probably by acting on hypodermal cells through EGL-15 activation. Our results support a juxtacrine/paracrine activity for LET-756 exerting locally its effect rather than through a large diffusion through the pseudocoelum.
ACKNOWLEDGEMENTS
We thank F. Birg and D. Marininchi for encouragements, C. Zemmour for statistical analyses, F. Roubin and M. Monleau for their help at some stages of the study. We are grateful to D. Thierry-Mieg for helpful discussions, M. Stern for the gift of the egl-17 construct, A. Fire for GFP expressing plasmids and the Caenorhabditis Genetics Center (CGC) for strains. The work has been supported by Inserm, Institut Paoli-Calmettes and grants from the Ligue Nationale Contre le Cancer (Label).