- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Food Science Journal
(Discontinued)
ISSN: 1874-2564 ― Volume 13, 2021
Antioxidant Activity Profiling of Acetonic Extract of Jamun (Syzygium cumini L.) Seeds in Different In-Vitro Models
Neha Yadav1, Ajay Pal1, *, Sonam Sihag1, Nagesh C.R1
Abstract
Background:
Syzygium cumini L., commonly known as Jamun, black-plum, and Indian blackberry, is one of the most widely distributed trees in India with booming medical benefits and possesses antioxidant, anticancer and anti-diabetic properties. It belongs to the family Myrtaceae. Despite countless phytochemicals, seeds are not consumed and are the waste part of Jamun fruit.
Objective:
The objective of this study was to evaluate the antioxidant capacity of phenolics from Jamun seeds against a bundle of oxidant moieties.
Methods:
The 50% acetone extract of Jamun seeds was investigated for in-vitro antioxidant profiling. Assays include free radical scavenging activity, metal chelation activity, hydroxyl radical scavenging activity, hydrogen peroxide scavenging activity, total antioxidant activity, total reducing power, nitric oxide scavenging activity, and lipid peroxidation inhibition activity.
Results:
The extract depicted maximum DPPH radical scavenging activity followed by ABTS radical scavenging activity. Hefty metal chelation and nitric oxide scavenging activity were recorded while lipid peroxidation, H2O2, and OH- scavenging activity was intermediate.
Conclusion:
Jamun seed showed ample antioxidant activity and certifies that it is the right candidate for exploitation as a source of natural antioxidants to counteract autoxidation-induced pathologies or diseases.
Article Information
Identifiers and Pagination:
Year: 2020Volume: 12
First Page: 3
Last Page: 8
Publisher Id: TOFSJ-12-3
DOI: 10.2174/1874256402012010003
Article History:
Received Date: 29/4/2020Revision Received Date: 1/9/2020
Acceptance Date: 7/9/2020
Electronic publication date: 25/11/2020
Collection year: 2020
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Biochemistry, CCS Haryana Agricultural University, Hisar-125 004, India; Tel: +91-9466534456, +91-8168668595; E-mail: ajaydrdo@rediffmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 29-4-2020 |
Original Manuscript | Antioxidant Activity Profiling of Acetonic Extract of Jamun (Syzygium cumini L.) Seeds in Different In-Vitro Models | |
1. INTRODUCTION
The cellular level of Reactive Oxygen Species (ROS) is regulated by the interaction of complex antioxidant types of machinery in living systems. These natural antioxidants are well known to curtail the adverse effects of free radicals and withstand oxidative damage in biological entities. Antioxidants prevent the oxidation process that can damage cells in the body. They prevent the living system by scavenging the free radicals interactively and harmoniously and doing this get themselves oxidized instead of cells. When the ROS level rises, the damage becomes cumulative and results in incapacitating oxidative stress [1Swanson CA. Vegetables, fruits, and cancer risk: The role of phytochemicals. Phytochemicals A New Paradigm CRC Press LLC, Boca Raton, FL 1998; 14: 1-2.]. Dietary antioxidants can suppress the harmful effects of oxidative stress. Presently, most of the commonly used antioxidants like butylated hydroxyl toluene (BHT), Butylated Hydroxyl Anisole (BHA), and tert-butyl hydroquinone (TBHQ) are manufactured synthetically [2Dutta S, Roy D, De A, Dutta C, Bhattacharya S. Antioxidant and free radical scavenging activity of Trigonella foenum-graecum L, Murraya koenigii, Coriandrum sativum and Centella asiatica. Turkish J Agr Food Sci Tech 2016; 4(4): 255-61.
[http://dx.doi.org/10.24925/turjaf.v4i4.255-261.520] ]. They are usually used to slow down the oxidative deterioration, but due to their probable toxic and carcinogenic effects, there has been increasing anguish over their use in fresh or processed foods [3Arabshahi-Delouee S, Urooj A. Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chem 2007; 102(4): 1233-40.
[http://dx.doi.org/10.1016/j.foodchem.2006.07.013] ]. Therefore, recent years have seen an increased surge of interest in finding naturally occurring antioxidants to substitute these synthetic antioxidants. As a result, the use of natural and safe antioxidants, especially of fruits and vegetable origin, has substantially increased among consumers and food scientists.
Epidemiological studies have persistently shown that consistent consumption of fruits and vegetables is strongly correlated with a lowered risk of developing chronic diseases such as cancer and cardiovascular disease [4Temple NJ. Antioxidants and disease: More questions than answers. Nutr Res 2000; 20(3): 449-59.
[http://dx.doi.org/10.1016/S0271-5317(00)00138-X] , 5Willett WC. Balancing life-style and genomics research for disease prevention. Science 2002; 296(5568): 695-8.
[http://dx.doi.org/10.1126/science.1071055] [PMID: 11976443] ]. Because of increased risk factors of various deadly diseases to humans, there has been a global trend towards the use of natural substances present in medicinal and dietary plants as the therapeutic antioxidants. It has been found in various studies that there is an inverse relationship between dietary intake of antioxidant-rich food/medicinal plants and the prevalence of human diseases. Wang et al. [6Wang H, Cao G, Prior RL. Total antioxidant capacity of fruits. J Agric Food Chem 1996; 44(3): 701-5.
[http://dx.doi.org/10.1021/jf950579y] ] affirmed that strawberry, orange, plum, red grape, kiwi fruit, and apple have healthy antioxidant activities. Studies conducted by Aqil et al. [7Aqil F, Gupta A, Munagala R, et al. Antioxidant and antiproliferative activities of anthocyanin/ellagitannin-enriched extracts from Syzygium cumini L. (Jamun, the Indian Blackberry). Nutr Cancer 2012; 64(3): 428-38.
[http://dx.doi.org/10.1080/01635581.2012.657766] [PMID: 22420901] ] have provided trustworthy evidence of Jamun's antioxidant potential and cancer-preventive properties. Worldwide, total production of Jamun is 13.5 million tonnes, out of which India contributes ~15.4% [8Raza A, Ali MU, Nisar T, Qasrani SA, Hussain R, Sharif MN. Proximate composition of Jamun (Syzygium cumini) fruit and seed. Digestion 2015; 2(4)]. Jamun is an evergreen tropical tree and is native to Bangladesh, India, Nepal, Pakistan, Sri Lanka, Philippines, and Indonesia. It is an endemic and important commercial fruit of India. It has also been recognized as a nutraceutical fruit due to the presence of robust antioxidant compounds such as ascorbic acid, anthocyanins, and total phenols. Jamun fruit has been found rich in carbohydrates, minerals, and vitamins. Its seed weighs 1-3 g, and an average-sized fruit contains 68-86 mg of pulp [9Margaret E, Shailaja AM, Venugopal Rao V. Evaluation of antioxidant activity in different parts of Syzygium cumini (Linn.). Int J Curr Microbiol Appl Sci 2015; 4(9): 372-9.]. Phytochemical analysis of Jamun seeds has revealed the presence of alkaloids, flavonoids, glycosides, phytosterols, saponins, tannins, and triterpenoids [10Kumar A, Ilavarasan R, Jayachandran T, et al. Phytochemicals investigation on a tropical plant, Syzygium cumini from Kattuppalayam, Erode district, Tamil Nadu, South India. Pak J Nutr 2009; 8(1): 83-5.
[http://dx.doi.org/10.3923/pjn.2009.83.85] ]. Its seeds contain albumen, fat, glycosides, jambosine, resin, ellagic acid, quercetin, gallic acid, as well as elements such as zinc, vanadium, sodium, and potassium [8Raza A, Ali MU, Nisar T, Qasrani SA, Hussain R, Sharif MN. Proximate composition of Jamun (Syzygium cumini) fruit and seed. Digestion 2015; 2(4)]. Despite these phytochemicals, seeds are not consumed and are the waste part of Jamun fruit. In our laboratory, we are continuously exploring new sources of phenolics compounds [11Pal A, Naika M, Khanum F, Bawa AS. In-vitro studies on the antioxidant assay profiling of Withania somnifera L. (Ashwagandha) Dunal root: Part 1. J Pharmacogn 2011; 3(20): 47-55.
[http://dx.doi.org/10.5530/pj.2011.20.10] , 12Pal A, Bhushan B, Narwal RK, Saharan V. Extraction and evaluation of antioxidant and free radical scavenging potential correlated with biochemical components of red rose petals. Iran J Sci Technol A 2018; 42(3): 1027-36.
[http://dx.doi.org/10.1007/s40995-016-0071-2] ]. In continuation, we have explored Jamun seed as a good source of phenolic compounds and have devised an effective extraction protocol to extract them (unpublished data). The present work, herein, represents the antioxidant potential of phenolics rich Jamun extract.
2. MATERIALS AND METHODS
2.1. Fruits and Chemicals
Jamun fruits used in present investigations were procured from the local market of Hisar. All the chemicals used in this investigation were of high-quality analytical grade and were purchased from M/s Sigma Chemicals Co., USA, Sisco Research Laboratory, Hi-Media, and E. Merck, Mumbai.
2.2. Methods
Seeds were separated from the pulp, chopped, shade dried in the dark, and ground to powder. Total phenolic compounds were extracted from the powder under the pre-optimized conditions using 25 ml/g volume of solvent (50% acetone), 193 rpm agitation rate, and 150 min extraction time (unpublished data). The extract was evaluated for its in-vitro antioxidant potential using an array of oxidants.
2.2.1. DPPH Radical Scavenging Assay
The extract/TPC was assayed in terms of its radical-scavenging ability using the stable radical, DPPH (1, 1 diphenyl–2–picrylhydrazyl), following the method of Blois [13Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958; 181(4617): 1199-200.
[http://dx.doi.org/10.1038/1811199a0] ].
DPPH radical scavenging activity was calculated using the formula given below –
DPPH scavenging activity (%) = [Ac-As/Ac] x 100
Where Ac is the absorbance of control solution and As is the absorbance of the test solution. IC50 (concentration of sample required to scavenge 50% free radicals) was calculated from a regression analysis. BHA was used as the standard antioxidant.
2.2.2. Metal Chelation Activity
The chelating effect of extracts on ferrous ions was determined according to the method of Dinis et al. [14Dinis TC, Maderia VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 1994; 315(1): 161-9.
[http://dx.doi.org/10.1006/abbi.1994.1485] [PMID: 7979394] ]. The results were expressed as IC50 calculated from a regression analysis. EDTA was used as a standard antioxidant.
2.2.3. ABTS Assay
The method used is based on the quenching of the most stable free cation (ABTS) decolorization assay [15Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic 1999; 26(9-10): 1231-7.
[http://dx.doi.org/10.1016/S0891-5849(98)00315-3] [PMID: 10381194] ]. The results were expressed as IC50 calculated from a regression analysis. BHA was used as a standard antioxidant.
2.2.4. Hydrogen Peroxide Scavenging Assay
The ability of sample extract to scavenge hydrogen peroxide was determined according to the modified method of Ruch et al. [16Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989; 10(6): 1003-8.
[http://dx.doi.org/10.1093/carcin/10.6.1003] [PMID: 2470525] ]. The results were expressed as IC50 calculated from a linear regression analysis. BHA was used as a standard antioxidant.
2.2.5. Hydroxyl Radical Scavenging Assay
The assay was performed following the method by Halliwell et al. [17Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 1987; 165(1): 215-9.
[http://dx.doi.org/10.1016/0003-2697(87)90222-3] [PMID: 3120621] ] with slight modifications. Percent inhibition was calculated from the control without samples under similar conditions. IC50 was calculated from a regression analysis. BHA was used as a standard antioxidant.
2.2.6. Total Antioxidant Activity
The assay was performed as described by Prieto et al. [18Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem 1999; 269(2): 337-41.
[http://dx.doi.org/10.1006/abio.1999.4019] [PMID: 10222007] ]. The antioxidant capacity was expressed as AO0.5AU (amount of extract that produces 0.5 absorbance units at selected wavelength). Gallic acid was used as a standard antioxidant.
2.2.7. Total Reducing Power
The total reducing power of the extract was determined by the method of Oyaizu [19Oyaizu M. Studies on products of browning reaction. Jap J Nutr Diet 1986; 44(6): 307-15.
[http://dx.doi.org/10.5264/eiyogakuzashi.44.307] ]. Increased absorbance of the reaction mixture indicates increased reducing power. The total reducing power was expressed as RP0.5AU (amount of extract that produces 0.5 absorbance units at selected wavelength). Gallic acid was used as a standard antioxidant.
2.2.8. Nitric Oxide Scavenging Activity
The procedure is based on the principle that sodium nitroprusside in aqueous solution at near-neutral pH spontaneously generates nitric oxide, which interacts with oxygen to produce nitrite ions that can be estimated using Griess reagent. Scavengers of nitric oxide compete with oxygen leading to decreased production of nitrite ions [20Sreejayan , Rao MN. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol 1997; 49(1): 105-7.
[http://dx.doi.org/10.1111/j.2042-7158.1997.tb06761.x] [PMID: 9120760] ]. The percent scavenging activity was calculated from the control without extract under similar conditions. IC50 was calculated from a regression analysis. Gallic acid was used as a standard antioxidant.
2.2.9. Lipid Peroxidation Inhibition Activity
A modified Thiobarbituric Acid Reactive Species (TBARS) assay [21Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95(2): 351-8.
[http://dx.doi.org/10.1016/0003-2697(79)90738-3] [PMID: 36810] ] was used to measure the lipid peroxide formed using egg yolk homogenate as a lipid-rich medium. The absorbance of the upper organic layer was measured at 532 nm. Inhibition of lipid peroxidation (%) was calculated using control without samples under similar conditions. IC50 was calculated from a regression analysis. BHA was used as a standard antioxidant.
3. RESULTS
The 50% acetone extract of Jamun seeds was investigated for the in-vitro antioxidant profile. Jamun seeds extract (JSE) was analyzed against a battery of oxidant moieties.
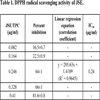
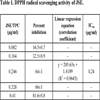
3.1. DPPH Radical Scavenging Assay
A dose-dependent increase in the quenching of free radicals was observed for JSE. In the present study, the IC50 value of JSE was calculated as 0.24 μg/ml (Table 1), indicating it as a good scavenger of DPPH radical. The JSE showed 64.21% DPPH scavenging at 0.25 µg/ml while BHA exhibited only 14.69% activity at the same concentration, thus proving JSE a better scavenger of free radicals than BHA, a well-known synthetic antioxidant.
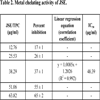
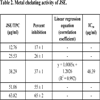
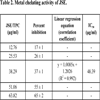
3.2. Metal Chelating Activity
A decrease in concentration-dependent color formation in the presence of the extract indicates iron-chelating potential. The metal chelating activity of JSE (IC50 value, 48.39 μg/ml) was calculated (Table 2) and compared with standard antioxidant – EDTA (IC50 value, 37.99 μg/ml) which was used as a positive control.
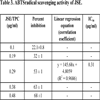
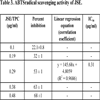
3.3. ABTS Radical Scavenging Assay
Results show that JSE possesses remarkable ABTS scavenging activity, and it increases as the concentration rises. In the present study, the IC50 value of JSE was 0.31 μg/ml (Table 3), indicating it a good scavenger of ABTS radicals.
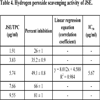
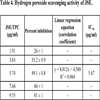
3.4. Hydrogen Peroxide Scavenging Activity
When compared with BHA (IC50 21.77 μg/ml) at equivalent concentrations, JSE exhibited better activity (IC50 5.67 μg/ml), as depicted in Table 4.
3.5. Hydroxyl Radical Scavenging Activity
The IC50 value from Table 5 depicts that the plant extract (5.75 μg/ml) is a better hydroxyl radical scavenger than the standard BHA (18.01 μg/ml). In contrast, Pal et al. (2011) showed that gallic acid had better hydroxyl radical scavenging activity than ashwagandha root extracts.
3.6. Total Antioxidant Activity
The extract was found to possess substantial TAA with AO0.5AU value of 5.37 µg/ml (Table 6) as compared with gallic acid (AO0.5AU 9.62 µg/ml).
3.7. Total Reducing Power
A rise in absorbance of the reaction mixture depicts an increase in reducing capacity due to the increased formation of the complex. The RP0.5 AU was found to be 3.10 µg/ml (Table 7), which was relatively more pronounced than that of gallic acid (5.47 µg/ml).
3.8. Nitric Oxide Radical Scavenging Assay
The nitric oxide radical scavenging activity of JSE (IC50 value, 37.01 μg/ml), as shown in Table 8, was compared with standard antioxidant – gallic acid (IC50 value, 33.2 μg/ml).
3.9. Lipid Peroxidation Inhibitory Activity
The decrease in MDA level by JSE illustrates its role as an antioxidant. The present study shows that JSE (IC50, 5.38 μg/ml) (Table 9) has more influential lipid peroxidation inhibitory activity as compared to BHA (IC50, 18.97 μg/ml).
4. DISCUSSION
4.1. DPPH Radical Scavenging Assay
The DPPH scavenging activity of the Jamun Seed Extract (JSE) was taken as a parameter to estimate its antioxidant potential. DPPH is a relatively stable radical, and bleaching of its absorption (515 nm) by a test compound is representative of its capacity to scavenge free radicals by donating a hydrogen atom to become a stable diamagnetic molecule. DPPH radical has the undeniable advantage of being unconcerned by the side reactions, i.e., enzyme inhibition and metal chelation [22Pal J, Ganguly S, Tahsin KS, Acharya K. In vitro free radical scavenging activity of wild edible mushroom, Pleurotus squarrosulus Mont: Singer 2010.]. Our results indicate distinct scavenging activity of the extract towards DPPH radicals in comparison with BHA. A high DPPH scavenging activity has also been reported in the acetonic extract of S. cumini leaves [23Kaneria M, Chanda S. Evaluation of antioxidant and antimicrobial capacity of Syzygium cumini L. leaves extracted sequentially in different solvents. J Food Biochem 2013; 37(2): 168-76.
[http://dx.doi.org/10.1111/j.1745-4514.2011.00614.x] ].
4.2. Metal Chelating Activity
Iron an acutely reactive metal and causes oxidative damage to lipids, proteins, and other cellular components. Metal chelating capacity is desirable to decrease the concentration of transition metals, causing lipid peroxidation. In this assay, EDTA had slightly higher chelating activity as compared to JSE, and similar results were found by Bholah et al. [24Bholah K, Ramful Baboolall D, Neergheen Bhujun VS. Antioxidant Activity of Polyphenolic Rich Moringa oleifera Lam. Extracts in Food Systems. J Food Biochem 2015; 39(6): 733-41.
[http://dx.doi.org/10.1111/jfbc.12181] ] in Moringa oleifera Lam. extracts and Pal et al. [12Pal A, Bhushan B, Narwal RK, Saharan V. Extraction and evaluation of antioxidant and free radical scavenging potential correlated with biochemical components of red rose petals. Iran J Sci Technol A 2018; 42(3): 1027-36.
[http://dx.doi.org/10.1007/s40995-016-0071-2] ] in red rose petal extracts.
4.3. ABTS Radical Scavenging Assay
The ABTS scavenging activity is taken as a parameter for desolvation of chain-breaking antioxidants in case of lipid peroxidation and antioxidant activity of hydrogen donating antioxidants [25Singh JP, Kaur A, Shevkani K, Singh N. Influence of jambolan (Syzygium cumini) and xanthan gum incorporation on the physicochemical, antioxidant and sensory properties of gluten‐free eggless rice muffins. Int J Food Sci Technol 2015; 50(5): 1190-7.
[http://dx.doi.org/10.1111/ijfs.12764] ]. The ABTS method is established as a rapid method for the determination of antioxidant activity and could be a convenient tool to screen samples and cultivars to obtain great content of natural antioxidants in foods. A high ABTS scavenging activity has also been found in Jamun fruit peel extract than standard compounds such as gallic acid, caffeic acid, sinapic acid, and quercetin [26Singh JP, Kaur A, Singh N, et al. In vitro antioxidant and antimicrobial properties of jambolan (Syzygium cumini) fruit polyphenols. Lebensm Wiss Technol 2016; 65: 1025-30.
[http://dx.doi.org/10.1016/j.lwt.2015.09.038] ].
4.4. Hydrogen Peroxide Scavenging Activity
The generation of even low levels of H2O2 in biological systems may be pernicious since naturally occurring iron complexes react with H2O2 to produce highly reactive hydroxyl radicals in a superoxide-driven ‘Fenton’ reaction [27Namiki M. Antioxidants/antimutagens in food. Crit Rev Food Sci Nutr 1990; 29(4): 273-300.
[http://dx.doi.org/10.1080/10408399009527528] [PMID: 2257080] ]. Pal et al. [11Pal A, Naika M, Khanum F, Bawa AS. In-vitro studies on the antioxidant assay profiling of Withania somnifera L. (Ashwagandha) Dunal root: Part 1. J Pharmacogn 2011; 3(20): 47-55.
[http://dx.doi.org/10.5530/pj.2011.20.10] ] showed that ascorbic acid had higher hydrogen peroxide scavenging activity than ashwagandha root extracts. Tchimene et al. [28Tchimene MK, Nwaehujor CO, Ezenwali M, Okoli CC, Iwu MM. Free radical scavenging activity of lupeol isolated from the methanol leaf extract of Crateva adansonii Oliv.(Capparidaceae). Int J Pharmacogn Phytochem Res 2016; 8: 419-26.] reported that leaf extract of Crateva adansonii had better hydrogen peroxide scavenging activity than ascorbic acid.
4.5. Hydroxyl Radical Scavenging Activity
This radical can cause strand damages in DNA, causing carcinogenesis, mutagenesis, and cytotoxicity. Moreover, hydroxyl radicals are capable of quick inception of the lipid peroxidation process by abstracting hydrogen atoms from unsaturated fatty acids [29Kappus H. Lipid peroxidation-mechanism and biological relevance. Free Radicals Food Addit 1991; 59-75., 30Aruoma OI. Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc 1998; 75(2): 199-212.
[http://dx.doi.org/10.1007/s11746-998-0032-9] [PMID: 32287334] ]. The hydroxyl radicals were produced in the present study by incubating ferric-EDTA with ascorbic acid and H2O2 at pH 7.4 and allowed to react with 2-deoxy-2-ribose to produce malondialdehyde (MDA)-like product. This compound then formed a pink chromogen upon heating with TBA at low pH.
4.6. Total Antioxidant Activity (TAA)
Since the assay is simple and unaffected by other antioxidant measurements commonly employed, its application has been expanded to plant extracts [18Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem 1999; 269(2): 337-41.
[http://dx.doi.org/10.1006/abio.1999.4019] [PMID: 10222007] ]. The assay is successfully used in the quantification of vitamin E in seeds. Pal et al. [11Pal A, Naika M, Khanum F, Bawa AS. In-vitro studies on the antioxidant assay profiling of Withania somnifera L. (Ashwagandha) Dunal root: Part 1. J Pharmacogn 2011; 3(20): 47-55.
[http://dx.doi.org/10.5530/pj.2011.20.10] , 12Pal A, Bhushan B, Narwal RK, Saharan V. Extraction and evaluation of antioxidant and free radical scavenging potential correlated with biochemical components of red rose petals. Iran J Sci Technol A 2018; 42(3): 1027-36.
[http://dx.doi.org/10.1007/s40995-016-0071-2] ], differently, stated that gallic acid had higher TAA than ashwagandha root and red rose petal extracts.
4.7. Total Reducing Power (TRP)
The reducing capacity of a compound can be estimated by the direct reduction of Fe3+ to Fe2+. The addition of free Fe3+ to the reduced product leads to the formation of intense Perl’s Prussian blue complex, which possesses strong absorbance at 700 nm. This method is often used as an indicator of electron-donating activity, which is an important mechanism for testing the antioxidant activity of plant extracts [31Yildirim A, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem 2001; 49(8): 4083-9.
[http://dx.doi.org/10.1021/jf0103572] [PMID: 11513714] ]. Many reports have affirmed that there is a direct correlation between antioxidant activities and reducing the power of certain plant extracts [31Yildirim A, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem 2001; 49(8): 4083-9.
[http://dx.doi.org/10.1021/jf0103572] [PMID: 11513714] , 32Oktay M, Gülçin İ, Küfrevioğlu Öİ. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. Lebensm Wiss Technol 2003; 36(2): 263-71.
[http://dx.doi.org/10.1016/S0023-6438(02)00226-8] ]. Our present results imply that JSE has the potential to donate electrons to reactive free radicals, hence, converting them to more stable non-reactive species and terminating the free radical chain reaction.
4.8. Nitric Oxide Radical Scavenging Assay
Nitric oxide is a dynamic pleiotropic inhibitor of physiological processes such as smooth muscle relaxation, neuronal signaling, inhibition of platelet aggregation, and regulation of cell-mediated toxicity [33Hagerman AE, Riedl KM, Jones GA, et al. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem 1998; 46(5): 1887-92.
[http://dx.doi.org/10.1021/jf970975b] [PMID: 29072454] ]. The excessive production of NO is associated with various diseases like adjuvant arthritis, cancer, etc. Our results are following those reported by Pal et al. [11Pal A, Naika M, Khanum F, Bawa AS. In-vitro studies on the antioxidant assay profiling of Withania somnifera L. (Ashwagandha) Dunal root: Part 1. J Pharmacogn 2011; 3(20): 47-55.
[http://dx.doi.org/10.5530/pj.2011.20.10] ], where ascorbic acid showed higher nitric oxide radical scavenging activity than different extracts of ashwagandha root. The mechanism involved in the scavenging activity of the extract may be associated with its phenolic compounds, as indicated in earlier studies [34Kumar S, Mishra A, Pandey AK. Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complement Altern Med 2013; 13(1): 120.
[http://dx.doi.org/10.1186/1472-6882-13-120] [PMID: 23721571] ].
4.9. Lipid Peroxidation Inhibitory Activity (LPIA)
Lipid peroxidation is a crucial biological consequence of oxidative cellular damage and aging. The polyunsaturated fatty acyl side chains, due to their susceptibility to oxidative damage in membrane phospholipids, pose a consistent threat to cellular integrity and functions [35Jain SK. Evidence for membrane lipid peroxidation during the in vivo aging of human erythrocytes. Biomembranes 1988; 937(2): 205-10.
[http://dx.doi.org/10.1016/0005-2736(88)90242-8] [PMID: 3337801] ]. In the process, cyclic peroxides, lipid peroxides, and cyclic end peroxides are produced, which ultimately get fragmented into aldehydes like malondialdehyde (MDA). MDA forms a pink chromogen with TBA that has a strong absorbance at 532 nm. JSE inhibited the amount of MDA generated in the egg homogenate model. Anjum et al. [36Anjum S, Gani A, Ahmad M, et al. Antioxidant and antiproliferative activity of walnut extract (Juglans regia L.) processed by different methods and identification of compounds using GC/MS and LC/MS technique. J Food Process Preserv 2017; 41(1): e12756.
[http://dx.doi.org/10.1111/jfpp.12756] ] also showed that walnut extracts could prevent oxidative deterioration of linoleic acid. It may be due to their polyphenolic compounds which protect the fatty acids from getting rancid.
CONCLUSION
In-vitro antioxidant studies depict that the radical scavenging capacity of JSE for DPPH radical is almost similar to ABTS radical and much higher than that of metal chelation activity and nitric oxide scavenging activity. Moderate scavenging activity was recorded against lipid peroxidation, H2O2, and OH- radicals. Overall, it is essential to evaluate more than one antioxidant assay when natural plant extracts are being evaluated for their antioxidant potential.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial, or otherwise.
ACKNOWLEDGEMENTS
The authors are thankful to the Head of the Department for providing necessary facilities to carry out the research work.
REFERENCES
| [1] | Swanson CA. Vegetables, fruits, and cancer risk: The role of phytochemicals. Phytochemicals A New Paradigm CRC Press LLC, Boca Raton, FL 1998; 14: 1-2. |
| [2] | Dutta S, Roy D, De A, Dutta C, Bhattacharya S. Antioxidant and free radical scavenging activity of Trigonella foenum-graecum L, Murraya koenigii, Coriandrum sativum and Centella asiatica. Turkish J Agr Food Sci Tech 2016; 4(4): 255-61. [http://dx.doi.org/10.24925/turjaf.v4i4.255-261.520] |
| [3] | Arabshahi-Delouee S, Urooj A. Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chem 2007; 102(4): 1233-40. [http://dx.doi.org/10.1016/j.foodchem.2006.07.013] |
| [4] | Temple NJ. Antioxidants and disease: More questions than answers. Nutr Res 2000; 20(3): 449-59. [http://dx.doi.org/10.1016/S0271-5317(00)00138-X] |
| [5] | Willett WC. Balancing life-style and genomics research for disease prevention. Science 2002; 296(5568): 695-8. [http://dx.doi.org/10.1126/science.1071055] [PMID: 11976443] |
| [6] | Wang H, Cao G, Prior RL. Total antioxidant capacity of fruits. J Agric Food Chem 1996; 44(3): 701-5. [http://dx.doi.org/10.1021/jf950579y] |
| [7] | Aqil F, Gupta A, Munagala R, et al. Antioxidant and antiproliferative activities of anthocyanin/ellagitannin-enriched extracts from Syzygium cumini L. (Jamun, the Indian Blackberry). Nutr Cancer 2012; 64(3): 428-38. [http://dx.doi.org/10.1080/01635581.2012.657766] [PMID: 22420901] |
| [8] | Raza A, Ali MU, Nisar T, Qasrani SA, Hussain R, Sharif MN. Proximate composition of Jamun (Syzygium cumini) fruit and seed. Digestion 2015; 2(4) |
| [9] | Margaret E, Shailaja AM, Venugopal Rao V. Evaluation of antioxidant activity in different parts of Syzygium cumini (Linn.). Int J Curr Microbiol Appl Sci 2015; 4(9): 372-9. |
| [10] | Kumar A, Ilavarasan R, Jayachandran T, et al. Phytochemicals investigation on a tropical plant, Syzygium cumini from Kattuppalayam, Erode district, Tamil Nadu, South India. Pak J Nutr 2009; 8(1): 83-5. [http://dx.doi.org/10.3923/pjn.2009.83.85] |
| [11] | Pal A, Naika M, Khanum F, Bawa AS. In-vitro studies on the antioxidant assay profiling of Withania somnifera L. (Ashwagandha) Dunal root: Part 1. J Pharmacogn 2011; 3(20): 47-55. [http://dx.doi.org/10.5530/pj.2011.20.10] |
| [12] | Pal A, Bhushan B, Narwal RK, Saharan V. Extraction and evaluation of antioxidant and free radical scavenging potential correlated with biochemical components of red rose petals. Iran J Sci Technol A 2018; 42(3): 1027-36. [http://dx.doi.org/10.1007/s40995-016-0071-2] |
| [13] | Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958; 181(4617): 1199-200. [http://dx.doi.org/10.1038/1811199a0] |
| [14] | Dinis TC, Maderia VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 1994; 315(1): 161-9. [http://dx.doi.org/10.1006/abbi.1994.1485] [PMID: 7979394] |
| [15] | Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic 1999; 26(9-10): 1231-7. [http://dx.doi.org/10.1016/S0891-5849(98)00315-3] [PMID: 10381194] |
| [16] | Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989; 10(6): 1003-8. [http://dx.doi.org/10.1093/carcin/10.6.1003] [PMID: 2470525] |
| [17] | Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 1987; 165(1): 215-9. [http://dx.doi.org/10.1016/0003-2697(87)90222-3] [PMID: 3120621] |
| [18] | Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem 1999; 269(2): 337-41. [http://dx.doi.org/10.1006/abio.1999.4019] [PMID: 10222007] |
| [19] | Oyaizu M. Studies on products of browning reaction. Jap J Nutr Diet 1986; 44(6): 307-15. [http://dx.doi.org/10.5264/eiyogakuzashi.44.307] |
| [20] | Sreejayan , Rao MN. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol 1997; 49(1): 105-7. [http://dx.doi.org/10.1111/j.2042-7158.1997.tb06761.x] [PMID: 9120760] |
| [21] | Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95(2): 351-8. [http://dx.doi.org/10.1016/0003-2697(79)90738-3] [PMID: 36810] |
| [22] | Pal J, Ganguly S, Tahsin KS, Acharya K. In vitro free radical scavenging activity of wild edible mushroom, Pleurotus squarrosulus Mont: Singer 2010. |
| [23] | Kaneria M, Chanda S. Evaluation of antioxidant and antimicrobial capacity of Syzygium cumini L. leaves extracted sequentially in different solvents. J Food Biochem 2013; 37(2): 168-76. [http://dx.doi.org/10.1111/j.1745-4514.2011.00614.x] |
| [24] | Bholah K, Ramful Baboolall D, Neergheen Bhujun VS. Antioxidant Activity of Polyphenolic Rich Moringa oleifera Lam. Extracts in Food Systems. J Food Biochem 2015; 39(6): 733-41. [http://dx.doi.org/10.1111/jfbc.12181] |
| [25] | Singh JP, Kaur A, Shevkani K, Singh N. Influence of jambolan (Syzygium cumini) and xanthan gum incorporation on the physicochemical, antioxidant and sensory properties of gluten‐free eggless rice muffins. Int J Food Sci Technol 2015; 50(5): 1190-7. [http://dx.doi.org/10.1111/ijfs.12764] |
| [26] | Singh JP, Kaur A, Singh N, et al. In vitro antioxidant and antimicrobial properties of jambolan (Syzygium cumini) fruit polyphenols. Lebensm Wiss Technol 2016; 65: 1025-30. [http://dx.doi.org/10.1016/j.lwt.2015.09.038] |
| [27] | Namiki M. Antioxidants/antimutagens in food. Crit Rev Food Sci Nutr 1990; 29(4): 273-300. [http://dx.doi.org/10.1080/10408399009527528] [PMID: 2257080] |
| [28] | Tchimene MK, Nwaehujor CO, Ezenwali M, Okoli CC, Iwu MM. Free radical scavenging activity of lupeol isolated from the methanol leaf extract of Crateva adansonii Oliv.(Capparidaceae). Int J Pharmacogn Phytochem Res 2016; 8: 419-26. |
| [29] | Kappus H. Lipid peroxidation-mechanism and biological relevance. Free Radicals Food Addit 1991; 59-75. |
| [30] | Aruoma OI. Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc 1998; 75(2): 199-212. [http://dx.doi.org/10.1007/s11746-998-0032-9] [PMID: 32287334] |
| [31] | Yildirim A, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem 2001; 49(8): 4083-9. [http://dx.doi.org/10.1021/jf0103572] [PMID: 11513714] |
| [32] | Oktay M, Gülçin İ, Küfrevioğlu Öİ. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. Lebensm Wiss Technol 2003; 36(2): 263-71. [http://dx.doi.org/10.1016/S0023-6438(02)00226-8] |
| [33] | Hagerman AE, Riedl KM, Jones GA, et al. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem 1998; 46(5): 1887-92. [http://dx.doi.org/10.1021/jf970975b] [PMID: 29072454] |
| [34] | Kumar S, Mishra A, Pandey AK. Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complement Altern Med 2013; 13(1): 120. [http://dx.doi.org/10.1186/1472-6882-13-120] [PMID: 23721571] |
| [35] | Jain SK. Evidence for membrane lipid peroxidation during the in vivo aging of human erythrocytes. Biomembranes 1988; 937(2): 205-10. [http://dx.doi.org/10.1016/0005-2736(88)90242-8] [PMID: 3337801] |
| [36] | Anjum S, Gani A, Ahmad M, et al. Antioxidant and antiproliferative activity of walnut extract (Juglans regia L.) processed by different methods and identification of compounds using GC/MS and LC/MS technique. J Food Process Preserv 2017; 41(1): e12756. [http://dx.doi.org/10.1111/jfpp.12756] |