- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Leukemia Journal
(Discontinued)
ISSN: 1876-8164 ― Volume 5, 2013
IL-4/CD40L Co-Stimulation Induces Long-Term Proliferation for CD10-Positive Germinal Center B Cell-Like Diffuse Large B-Cell Lymphoma
Harumi Kato1, 2, 3, Yoshitoyo Kagami11, 3, Masao Nakagawa1, Sivasundaram Karnan1, Yasushi Yatabe4, Shigeo Nakamura4, 5, Yasuo Morishima3 , Masao Seto1, 2, *
Abstract
Diffuse large B cell lymphoma (DLBCL) is a heterogeneous entity and the explicit mechanism of lymphomagenesis remains to be elucidated. CD40 ligand (CD40L, CD154) and interleukin-4 (IL-4) are important factors regulating B lymphocyte proliferation and differentiation; however, little is known about the effects of these factors on lymphomagenesis in DLBCL. In this study, we investigated the effects of these factors on DLBCL B cell proliferation. Normal (n=2) and DLBCL (n=10) B cells were cultured with a system containing IL-4 and CD40L-expressing NIH3T3 (CD40L+3T3). Normal and tumor cells from six patients stopped growing within three weeks. Tumor cells, obtained from four patients with extranodal lesions, showed continuous proliferation for more than two months. Two cell lines were established from these cells. The cell lines were derived from gastric tissues showing CD10+ germinal center B-cell (GCB) phenotype. Removing IL-4 from the system or adding anti-CD40L blocking antibody inhibited the cell proliferation. An in vitro transwell assay showed that direct contact with CD40L+3T3 was required for the proliferation. Our findings suggested that CD40L and IL-4 co-stimulation could induce long-term proliferation in B cells derived from some CD10+ GCB-like DLBCL patients with the same characteristics, and that direct stimulation from tumor microenvironment is important for the cell growth. These cell lines may be useful for investigating the growth mechanism of some type of DLBCL, and should provide new insights concerning pathogenesis of DLBCL.
Article Information
Identifiers and Pagination:
Year: 2010Volume: 3
First Page: 60
Last Page: 68
Publisher Id: TOLEUKEMIAJ-3-60
DOI: 10.2174/1876816401003010060
Article History:
Received Date: 14/4/2010Revision Received Date: 26/7/2010
Acceptance Date: 29/7/2010
Electronic publication date: 30/09/2010
Collection year: 2010
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http: //creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Division of Molecular Medicine, Aichi Cancer Center Research Institute, 1-1 Kanokoden, Chikusa-ku, Nagoya, Aichi 464-8681, Japan; Tel: +81-52-764-2982;Fax: +81-52-764-2982; E-mail: mseto@aichi-cc.jp
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 14-4-2010 |
Original Manuscript | IL-4/CD40L Co-Stimulation Induces Long-Term Proliferation for CD10-Positive Germinal Center B Cell-Like Diffuse Large B-Cell Lymphoma | |
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin’s lymphoma (NHL), accounting for 30-40% of newly diagnosed tumors of lymphoid tissues [1Coiffier B. Diffuse large cell lymphoma Curr Opin Oncol 2001; 13: 325-4.]. DLBCL is etiologically, pathologically and cytogenetically a heterogeneous disease. Gene expression profiling has identified three subtypes of DLBCL: germinal-center B cell-like, activated B cell-like, and type 3 diffuse large B-cell lymphoma [2Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling Nature 2000; 403: 503-11., 3Rosenwald A, Wright G, Chan WC, et al. Lymphoma/Leukemia Molecular Profiling Project.The use of molecular profiling to predict survival after chemotherapy for diffuse large B-cell lymphoma N Engl J Med 2002; 346: 1937-47.]. These three subtypes show different clinical outcomes which may require different therapeutic approaches. Although many efforts have been made to improve survival [4Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma N Engl J Med 2002; 346: 235-42., 5Fu K, Weisenburger DD, Choi WW, et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma J Clin Oncol 2008; 26: 4587-94.], some patients with relapse show poor prognosis. To stratify appropriate treatment strategies and develop new potential therapies, an understanding of the pathological and molecular mechanism of the disease is essential.
CD40 is a type-1 transmembrane protein of the tumor necrosis factor receptor superfamily, and is expressed on virtually all mature B lymphocytes and various B lineage neoplasms. Stimulation of CD40 antigen is thought to play an important role in B-cell proliferation, survival, differentiation and function [6van Kooten C, & Banchereau J. CD40-CD40 ligand J Leukoc Biol 2000; 67: 2-17.], as well as malignant B-cell proliferation [7Kluin-Nelemans HC, Beverstock GC, Mollevanger P, et al. Proliferation and cytogenetic analysis of hairy cell leukemia upon stimulation via the CD40 antigen Blood 1994; 84: 3134-41., 8Eliopoulos AG, & Young LS. The role of the CD40 pathway in the pathogenesis and treatment of cancer Curr Opin Pharmacol 2004; 4: 360-7.]. The CD40 ligand (CD40L, or CD154) is expressed primarily on the surface of activated CD4+ T lymphocytes [9Armitage RJ, Fanslow WC, Strockbine L, et al. Molecular and biological characterization of a murine ligand for CD40 Nature 1992; 357: 80-2., 10Roy M, Waldschmidt T, Aruffo A, et al. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells J Immunol 1993; 151: 2497-510.]. Additionally, CD40L expression has been shown in several types of lymphoma [11Younes A, Kadin ME. Emerging applications of the tumor necrosis factor family of ligands and receptors in cancer therapy J Clin Oncol 2003; 21: 3526-4., 12Pham LV, Tamayo AT, Yoshimura LC, et al. A CD40 signalosome anchored in lipid rafts leads to constitutive activation of NF-kappaB and autonomous cell growth in B cell lymphomas Immunity 2002; 16: 37-50.]. It was reported that CD40L expression in B-cell lymphomas may contribute to proliferation of lymphoma cells through activation of intrinsic CD40 [12Pham LV, Tamayo AT, Yoshimura LC, et al. A CD40 signalosome anchored in lipid rafts leads to constitutive activation of NF-kappaB and autonomous cell growth in B cell lymphomas Immunity 2002; 16: 37-50., 13Jost PJ, Ruland J. Aberrant NF-kappaB signaling in lymphoma: mechanisms, consequences, and therapeutic implications Blood 2007; 109: 2700-7.]. Thus, CD40/CD40L has been suggested as a system contributing to tumor pathogenesis.
IL-4 is thought to increase CD40 antigen expression [14Vallé A, Zuber CE, Defrance T, et al. Activation of human B lymphocytes through CD40 and interleukin 4 Eur J Immunol 1989; 19: 1463-7.], and CD40L and IL-4 co-stimulation has resulted in long-term B-cell proliferation in vitro [15Banchereau J, de Paoli P, Vallé A, et al. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40 Science 1991; 251: 70-2., 16Wiesner M, Zentz C, Mayr C, et al. Conditional immortalization of human B cells by CD40 ligation PLoS One 2008 Jan 23; 23: e1464 [about 13 screens]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2180193/ [[cited 2008 January 23]];]. CD40L and IL-4 co-stimulation is also reported to promote short-term proliferations of mantle cell lymphoma and a proportion of hairy cell leukemia B cells [7Kluin-Nelemans HC, Beverstock GC, Mollevanger P, et al. Proliferation and cytogenetic analysis of hairy cell leukemia upon stimulation via the CD40 antigen Blood 1994; 84: 3134-41., 17Castillo R, Mascarenhas J, Telford W, et al. Proliferative response of mantle cell lymphoma cells stimulated by CD40 ligation and IL-4 Leukemia 2000; 14: 292-8.], as well as prolonging proliferations of follicular lymphoma B cells (for up to eight weeks) [18Johnson PW, Watt SM, Betts DR, et al. Isolated follicular lymphoma cells are resistant to apoptosis and can be grown in vitro in the CD40/stromal cell system Blood 1993; 82: 1848-57.]. However, little is known about the role of CD40L and IL-4 co-stimulation on DLBCL B-cell proliferation. Herein, we investigated the proliferative ability of DLBCL B cells using a CD40L and IL-4 co-stimulation culture system.
MATERIALS AND METHODOLOGY
Characteristic of Patients and Sample Preparation
This study was approved by the institutional review board of the Aichi Cancer Center. Mononuclear cells from pathologic lymph node or tissue specimens (ten cases), and from peripheral blood (two healthy donors), were obtained (Table 1) after written informed consent in accordance with the Declaration of Helsinki. Mononuclear cells were isolated by the addition of Ficoll and density centrifugation. All patients were diagnosed on the basis of morphological and immunohistochemical findings in accordance with WHO criteria. Categorization as germinal center B cell-like (GCB) or non-germinal center B cell-like (non-GCB) was performed according to the algorithm using the Hans classification [19Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray Blood 2004; 103: 275-82.].
Culture Conditions and Reagents
The cells were cultured with RPMI 1640 medium containing antibiotics, 10% fatal calf serum (PAA Laboratories GmbH, Austria), 100 nM sodium selenite (Wako Pure Chemical Industries, Osaka, JP), 1 µg/ml cyclosporin A (CsA; Wako Pure Chemical Industries, Osaka, JP) and 2 ng/ml recombinant human IL-4 (PeproTech Inc, Rocky Hill, NJ). NIH3T3 (NIH Swiss mouse embryonic fibroblast cell line) transfected with CD40L (CD40L+3T3), which was kindly supplied by Dr. Lee M. Nadler (Dana-Farber Cancer Institute, Boston, MA, USA) [20Schultze JL, Cardoso AA, Freeman GJ, et al. Follicular lymphomas can be induced to present alloantigen efficiently a conceptual model to improve their tumor immunogenicity Proc Natl Acad Sci USA 1995; 92: 8200-4.], was used for stimulating feeder cells and co-cultured with B cells.
CD40L+3T3 was cultured for one to three days at 1×105cells/well using a 12-well plate and 2×104 cells/well using a 96-well plate, and then irradiated (100 Gy) on the day B cells were plated. Since one EBV-infected cell is found in about 104-106 B cells, unseparated mononuclear cells from peripheral blood were plated in different numbers per microculture to established EBV-free CD40-stimulated B cell cultures [16Wiesner M, Zentz C, Mayr C, et al. Conditional immortalization of human B cells by CD40 ligation PLoS One 2008 Jan 23; 23: e1464 [about 13 screens]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2180193/ [[cited 2008 January 23]];]. The cells were cultured on CD40L+3T3 in the presence of interleukin-4 and CsA in a humidified atmosphere at 37°C with 5% CO2 in air for three to seven days [16Wiesner M, Zentz C, Mayr C, et al. Conditional immortalization of human B cells by CD40 ligation PLoS One 2008 Jan 23; 23: e1464 [about 13 screens]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2180193/ [[cited 2008 January 23]];]. The cell number was counted using the trypan blue dye exclusion test in each well, and the cells were transferred to new stimulating feeder cells at the same conditions and with the same concentration of B cells.
Immunophenotyping
Cell surface antigen expression of B-cell marker was studied by flow cytometry using FITC, PE, PerCP or APC labeled CD5 (Becton, Dickinson and Company; BD, Mountain View, CA, USA), CD10 (BD), CD19 (BD), CD20 (BD), and surface immunoglobulin (sIg) (BD). The CD40L expression of feeder cells and established cell lines was evaluated with anti-CD40L antibody (monoclonal antibody CD154, murine IgG1, TRAP-1 clone; Immunotech, a Beckman Coulter Company, France) using FITC-labeled sIg (goat, DakoCytomation, Glostrup, Denmark) as a secondary antibody. The events acquired by the cytometer varied from sample to sample, because the events depend on the size of the culture system.
Transwell Culture
CD40L+3T3 cells were plated at 7×104/well using a 12-well plate (Transwell, Costar) on day -1. The medium was changed to 1.5 ml of fresh medium on day 0, and the bottom cup with a 0.4-ìm diameter microporous membrane was put in place. B cells were inoculated into the inner cup with 0.5 ml of cell suspensions containing 0.5×105 cells/ml. The positive control consisted of three conditions: culture with the inner cup without feeder cells, culture without the inner cup with CD40L+3T3 cells, and culture without the inner cup with NIH3T3 untransfected with CD40L (CD40L-3T3) cells. B cells, cultured without the inner cup, were plated with 2 ml of cell suspensions containing 0.5×105 cells/ml using a 12-well plate. B-cell number was counted every four days using the trypan blue dye exclusion test in each well, and the cells were transferred to new plates at the same conditions and with the same concentration of B cells. The culture was continued until day 8. Each condition was repeated in duplicate, and the cell number was counted two times in each well.
DNA Extraction and 4×44K Oligo Array Comparative Genomic Hybridization (CGH) Analysis
DNA was extracted from frozen tissue samples using proteinase K treatment and phenol-chloroform extraction. Oligo Array CGH analysis was performed using the Agilent Human Genomic Microarray Kit 4×44K (Agilent Technologies). This high-resolution 60-mer oligonucleotide-based microarray was used in this study and contains about 44,000 probes with an average spatial resolution of 35 kb (Agilent Technologies, Santa Clara, CA). Genomic DNA from the patient and normal male reference DNA were digested with AluI and RsaI (Promega Corporation) and labeled using an Agilent Genomic DNA Labeling kit according to manufacturer’s instructions. Patient and reference DNA were labeled with Cy3 and Cy5, respectively. Labeled patient DNA and reference DNA were co-hybridized to arrays for 24 hr at 65°C in a rotating oven (Agilent Technologies) at 20 rpm. Washing was performed according to the Agilent protocol. Arrays were analyzed using an Agilent Microarray Scanner and Agilent Feature Extraction software (9.1). Results were presented by Agilent CGH Analytics software (v3.4.27).
RESULTS
CD40L and IL-4 Co-Stimulation Induce Normal B Lymphocyte Proliferation In Vitro
Peripheral blood mononuclear cells from two healthy donors were cultured on the stimulator cells of CD40L+3T3 with IL-4 containing medium. The cells were plated with 2 ml of cell suspensions containing 0.5×106/ml using a 12-well plate, and with 200 ìl of cell suspensions containing 1.25×105/ml, 2.5×105/ml, 5.0×105/ml or 10.0×105/ml using a 96-well plate. Since the best growth curve was obtained using cell suspensions containing an initial concentration of 1.25×105/ml with a 96-well plate (data not shown), we chose this condition for further experiments. For the first several days, the cells formed a small cluster on CD40L+3T3 cells, and after a further five to seven days, most cells became large clusters. The cells, adjusted to a concentration of 1.25×105/ml, were restimulated every 5 to 7 days with fresh stimulator cells. The cells proliferated to about 110 to 120-fold until day 20. Wiesner et al. [16Wiesner M, Zentz C, Mayr C, et al. Conditional immortalization of human B cells by CD40 ligation PLoS One 2008 Jan 23; 23: e1464 [about 13 screens]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2180193/ [[cited 2008 January 23]];] showed that cultures stimulated with CD40L-expressing murine fibroblasts in the presence of IL-4 can proliferate for more than 900 days. However, the use of our culture system with CD40L+3T3 cells and IL-4 resulted in proliferation for about three weeks, and we observed that cell growth through proliferation rapidly ceased (Fig. 1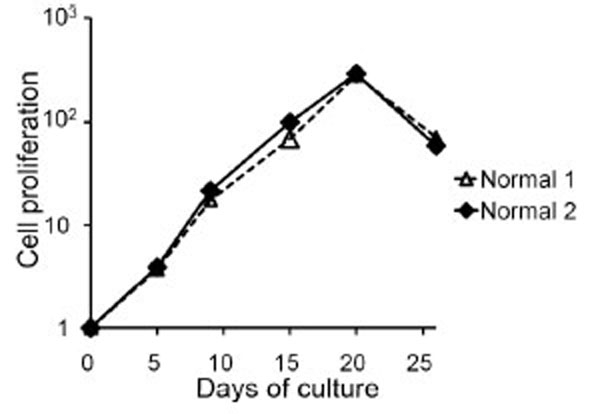 ).
).
Co-Stimulation of CD40L and IL-4 Induces DLBCL Cell Proliferation In Vitro
Mononuclear cells from 10 DLBCL samples (five stomach, three lymph node and two breast) were plated with 200 ìl of cell suspensions containing 1.25×105/ml on a 96-well plate using the stimulator cells of CD40L+3T3 with IL-4 containing medium. Of the ten samples, tumor cells from six patients stopped growing within three weeks. The cells from the remaining four patients have been cultured with continuous proliferation for more than two months, and cell lines were established from two of these cell groups (GDLB-GCB1 and GDLB-GCB2). The cell lines, derived from gastric tissue, showed proliferation for more than three month. The doubling time of the cell lines GDLB-GCB1 (from UPN (unique patient number)1 patient) and GDLB-GCB2 (from UPN2 patient) was 2.9 and 1.7 days, respectively (Fig. 2a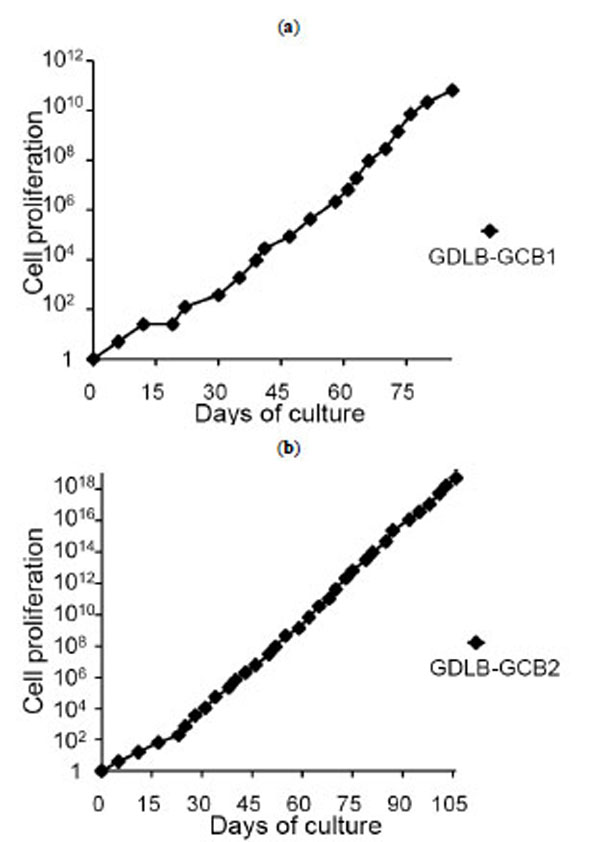 , b
, b ). These cell lines showed pathological characteristics of bcl2- and CD10+ germinal center B-cell (GCB)-like DLBCL. Phenotypical analysis of the original samples and established cell lines demonstrated the positive expression of CD10, CD19, CD20 and sIgG, and the negative or weakly positive expression of CD40L (Fig. 3
). These cell lines showed pathological characteristics of bcl2- and CD10+ germinal center B-cell (GCB)-like DLBCL. Phenotypical analysis of the original samples and established cell lines demonstrated the positive expression of CD10, CD19, CD20 and sIgG, and the negative or weakly positive expression of CD40L (Fig. 3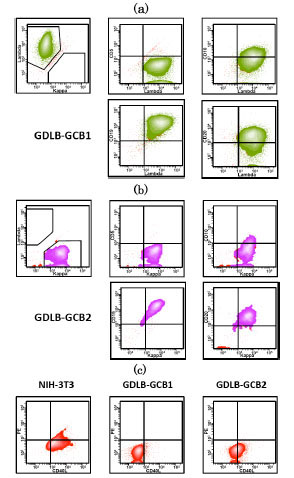 ). UPN 6 and UPN 10 ceased to proliferate within 2.5 to 3 months.
). UPN 6 and UPN 10 ceased to proliferate within 2.5 to 3 months.
Proliferation Analysis in the Presence or Absence of Anti-CD40L Antibody and Cytokines
We next examined whether the growth of two established cell lines depended on IL-4 and CD40L. The method of CD40L blocking was conducted as our previous experiments that were used for the other antibodies (Kagami et al.) [21Kagami Y, Tobinai K, Kinoshita T, et al. Novel interleukin-2 dependent T-cell line derived from adult T-cell leukemia not associated with human T-cell leukemia virus type I Jpn J Cancer Res 1993; 84: 371-8.]. Removal of IL-4 from the system containing the stimulator cells of CD40L+3T3 with IL-4 containing medium resulted in growth inhibition. Proliferation of B cells from the cell lines was also inhibited in medium only or medium containing IL-4 (Fig. 4a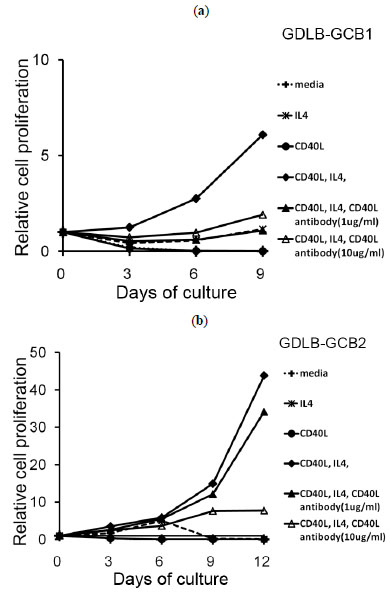 , b
, b ). When anti-CD40L blocking antibody was added to this culture system, the proliferation of these cell lines was inhibited (Fig. 4a
). When anti-CD40L blocking antibody was added to this culture system, the proliferation of these cell lines was inhibited (Fig. 4a , b
, b ,). Proliferation of the cells from GDLB-GCB2 was inhibited in a concentration-dependent manner (Fig.b
,). Proliferation of the cells from GDLB-GCB2 was inhibited in a concentration-dependent manner (Fig.b ).
).
Proliferation Analysis with Transwell Culture
The use of an in vitro transwell assay, which prevents direct cell-to-cell contact, allows confirmation of whether direct contact with CD40L+3T3 cells is required for proliferation of the B-cell lines. The cells inoculated into the inner cup with the transwell assay showed cell growth arrest. Cells of GDLB-GCB2 also stopped growing when the cells were cultured with CD40L-3T3 (Fig. 5b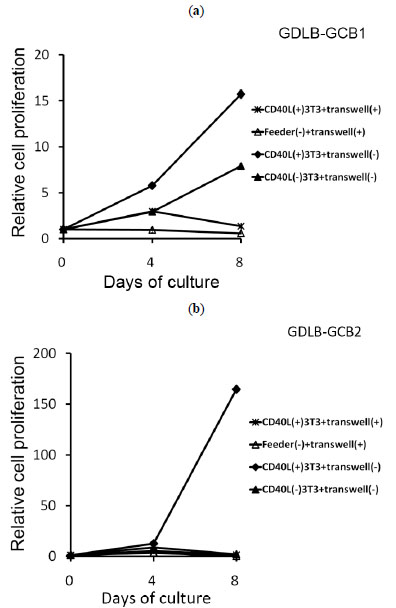 ). On the other hand, cells of GDLB-GCB1 stimulated with CD40L-3T3 showed cell growth on day 8 (Fig. 5a
). On the other hand, cells of GDLB-GCB1 stimulated with CD40L-3T3 showed cell growth on day 8 (Fig. 5a ). When these cell lines were cultured with CD40L-3T3 cells in a 96-well plate, the two cell lines stopped growing as expected.
). When these cell lines were cultured with CD40L-3T3 cells in a 96-well plate, the two cell lines stopped growing as expected.
Array CGH Analysis
Array CGH analysis revealed that the established cell lines showed the identical genomic imbalances as the original samples, indicating that the established cell lines and original samples were derived from the identical clones (Fig. 6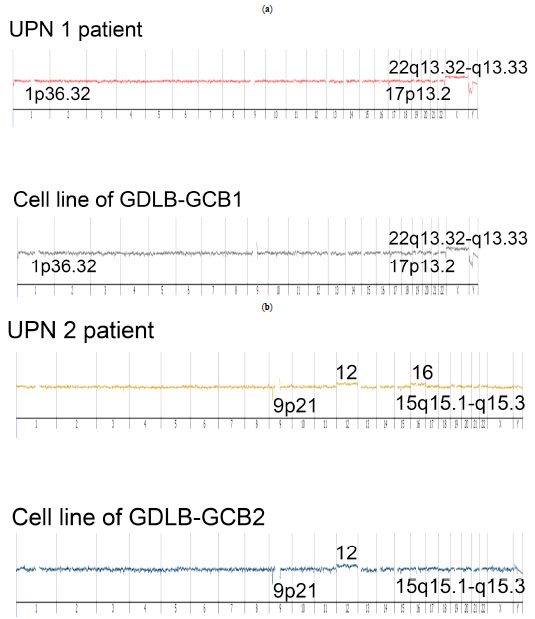 ). However, the established cell lines did not have common distinctive genomic imbalances. Array CGH data of the short-term proliferating cells could not be obtained because of the cell death.
). However, the established cell lines did not have common distinctive genomic imbalances. Array CGH data of the short-term proliferating cells could not be obtained because of the cell death.
DISCUSSION
Established Cell Lines
In this report, we demonstrated that IL-4 and CD40L co-stimulation induced short-term proliferation in DLBCL B cells, and some type of DLBCL B cells showed a long-term proliferation. Interestingly, the three cases including the established two cell lines had common clinicopathological features, presenting with a local extra nodal lesion and a bcl2- CD10+ GCB-like DLBCL phenotype. Moreover, removing IL-4 from the culture system or adding anti-CD40L blocking antibody to the system inhibited proliferation of the cell lines. These results suggested that CD40L and IL-4 co-stimulation played a critical role in long-term proliferation for some type of DLBCL in vitro. However, it is possible that more cell lines could have been established under different conditions. It is also conceivable that tumor cell purity influenced the results.
CD40/CD40L Signaling in DLBCL B-Cell Proliferation
The biological behavior, tumor progression and prognosis of patients may be influenced by the tumor microenvironment for some types of malignant lymphomas [22Kagami Y, Jung J, Choi YS, et al. Establishment of a follicular lymphoma cell line (FLK-1) dependent on follicular dendritic cell-like cell line HK Leukemia 2001; 15: 148-56.-24Monti S, Savage KJ, Kutok JL, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response Blood 2005; 105: 1851-61.]. CD40L is expressed mainly in activated CD4+ T lymphocytes. CD40L binds to CD40 of normal B cells, and induces growth or survival signals of B cells [15Banchereau J, de Paoli P, Vallé A, et al. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40 Science 1991; 251: 70-2., 16Wiesner M, Zentz C, Mayr C, et al. Conditional immortalization of human B cells by CD40 ligation PLoS One 2008 Jan 23; 23: e1464 [about 13 screens]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2180193/ [[cited 2008 January 23]];]. CD40L expression has also been shown in several types of B-cell lymphomas [11Younes A, Kadin ME. Emerging applications of the tumor necrosis factor family of ligands and receptors in cancer therapy J Clin Oncol 2003; 21: 3526-4., 12Pham LV, Tamayo AT, Yoshimura LC, et al. A CD40 signalosome anchored in lipid rafts leads to constitutive activation of NF-kappaB and autonomous cell growth in B cell lymphomas Immunity 2002; 16: 37-50.]. Pham et al. reported that endogenously expressed CD40L in aggressive B-cell lymphomas binds to the CD40 receptor within cell membrane lipid rafts, and that this CD40 signalosome constitutively activates the canonical NF-êB signaling pathway [12Pham LV, Tamayo AT, Yoshimura LC, et al. A CD40 signalosome anchored in lipid rafts leads to constitutive activation of NF-kappaB and autonomous cell growth in B cell lymphomas Immunity 2002; 16: 37-50.]. In the present study, endogenous CD40L expression was negative in GDLB-GCB1, and weakly positive in GDLB-GCB2. Removing CD40L+3T3 feeder cells from the CD40L and IL-4 co-stimulation system or adding anti-CD40L blocking antibody to the system inhibited proliferation of these cell lines. An in vitro transwell assay also showed cell growth arrest. These
experiments suggested that direct CD40L stimulation from the tumor microenvironment, but not endogenously expressed CD40L, is important for the proliferation of some type of DLBCL.
Effect of IL-4 on DLBCL Proliferation
IL-4 is a pleiotropic cytokine regulating differentiation and growth of normal B cells. IL-4 is known to be a potent survival factor for many cell types, and the effect on neoplastic B cells is different for each lymphoma type [25Paul WE, et al. Interleukin-4 a prototypic immunoregulatory lymphokine Blood 1991; 77: 1859-70.-28Kadoch C, Dinca EB, Voicu R, et al. Pathologic correlates of primary central nervous system lymphoma defined in an orthotopic xenograft model Clin Cancer Res 2009; 15: 1989-97.]. Lu et al. [29Lu X, Nechushtan H, Ding F, et al. Distinct IL-4-induced gene expression, proliferation, and intracellular signaling in germinal center B-cell-like and activated B-cell-like diffuse large-cell lymphomas Blood 2005; 105: 2924-32.] reported that in GCB-like DLBCL, IL-4 induces expression of its known target genes by activation of STAT6, resulting in cell proliferation. In contrast, in ABC-like DLBCL, IL-4 activates the Akt pathway and results in a decrease of cell proliferation by cell-cycle arrest [29Lu X, Nechushtan H, Ding F, et al. Distinct IL-4-induced gene expression, proliferation, and intracellular signaling in germinal center B-cell-like and activated B-cell-like diffuse large-cell lymphomas Blood 2005; 105: 2924-32.]. In our study, IL-4 was also an important factor for promoting proliferation of the established cell lines; however, the stimulation of IL-4 in the absence of CD40L did not show any significant cell growth. For some GCB-like DLBCL patients, CD40L and IL-4 co-stimulation might be associated with lymphoma cell proliferation.
Genetic Characterization and Clinicopathological Features of the Established Cell Lines
Genetic Characterization of the Established Cell Lines
Array CGH analysis showed genetic alteration (deletion of 1p36) in GDLB-GCB1. A recent report revealed a new subtype of FL, which has a characteristic clinicopathological feature of a predominantly diffuse infiltration pattern. It also shows characteristic genetic features with a unifying chromosomal deletion in 1p36 and lack of t(14;18), typical for FL [30Katzenberger T, Kalla J, Leich E, et al. A distinctive subtype of t(14,18)-negative nodal follicular non-Hodgkin lymphoma characterized by a predominantly diffuse growth pattern and deletions in the chromosomal region 1p36 Blood 2009; 113: 1053-61.]. The report showed that genetic alteration existed in 27 of 29 cases [30Katzenberger T, Kalla J, Leich E, et al. A distinctive subtype of t(14,18)-negative nodal follicular non-Hodgkin lymphoma characterized by a predominantly diffuse growth pattern and deletions in the chromosomal region 1p36 Blood 2009; 113: 1053-61.]. It is interesting to find that the same genetic aberration were present in our established cell line.
Clinicopathological Features of the Established Cell Lines
In relation to the distinct subtype of FL, the aforementioned report also revealed that many patients had some clinically unique characteristics: low clinical stage, large localized inguinal tumors, and an immunophenotype with frequent expressions of CD10, BCL6 and CD23. The immunophenotype was in accordance with a germinal center B-cell phenotype [30Katzenberger T, Kalla J, Leich E, et al. A distinctive subtype of t(14,18)-negative nodal follicular non-Hodgkin lymphoma characterized by a predominantly diffuse growth pattern and deletions in the chromosomal region 1p36 Blood 2009; 113: 1053-61.]. In our series, three cases, all representing long-term proliferation, were GCB-like DLBCL and had local extranodal lesions. They also exhibited the same pathological characteristics of bcl2- and CD10+ GCB-like DLBCL, and proliferated in the same system. These results suggested that this group might form a unique entity. Further study is needed to clarify if this type of DLBCL warrants classification as a unique subgroup.
In summary, we demonstrated that CD40L and IL-4 co-stimulation induced the long-term proliferation of some type of DLBCL. Moreover, direct contact with CD40L in the tumor microenvironment is important for cell proliferation. We established DLBCL cell lines stimulated with CD40L and IL-4. These cell lines may be useful for investigating the growth mechanism of GCB-like DLBCL in vitro and might provide new insights regarding the pathogenesis of the lymphoma.
CONFLICT OF INTEREST
The authors indicate no potential conflicts of interest.
ACKNOWLEDGEMENTS
The authors thank Mako Hagino and Hiroshi Hamajima for their excellent laboratory work. We would also like to say a special thank to Dr. Shinobu Tsuzuki, Dr. Kennosuke Karube and Dr. Keiichiro Honma for their helpful and inspiring discussions.
This study was supported partially by Ministry of Health, Labor and Welfare, Ministry of Education, Culture, Sports, Science and Technology, Japan Society for the Promotion of Science, Foundation for Promotion of Cancer Research, and Wella Award from the Japan Leukemia Research Fund.
REFERENCES
| [1] | Coiffier B. Diffuse large cell lymphoma Curr Opin Oncol 2001; 13: 325-4. |
| [2] | Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling Nature 2000; 403: 503-11. |
| [3] | Rosenwald A, Wright G, Chan WC, et al. Lymphoma/Leukemia Molecular Profiling Project.The use of molecular profiling to predict survival after chemotherapy for diffuse large B-cell lymphoma N Engl J Med 2002; 346: 1937-47. |
| [4] | Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma N Engl J Med 2002; 346: 235-42. |
| [5] | Fu K, Weisenburger DD, Choi WW, et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma J Clin Oncol 2008; 26: 4587-94. |
| [6] | van Kooten C, & Banchereau J. CD40-CD40 ligand J Leukoc Biol 2000; 67: 2-17. |
| [7] | Kluin-Nelemans HC, Beverstock GC, Mollevanger P, et al. Proliferation and cytogenetic analysis of hairy cell leukemia upon stimulation via the CD40 antigen Blood 1994; 84: 3134-41. |
| [8] | Eliopoulos AG, & Young LS. The role of the CD40 pathway in the pathogenesis and treatment of cancer Curr Opin Pharmacol 2004; 4: 360-7. |
| [9] | Armitage RJ, Fanslow WC, Strockbine L, et al. Molecular and biological characterization of a murine ligand for CD40 Nature 1992; 357: 80-2. |
| [10] | Roy M, Waldschmidt T, Aruffo A, et al. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells J Immunol 1993; 151: 2497-510. |
| [11] | Younes A, Kadin ME. Emerging applications of the tumor necrosis factor family of ligands and receptors in cancer therapy J Clin Oncol 2003; 21: 3526-4. |
| [12] | Pham LV, Tamayo AT, Yoshimura LC, et al. A CD40 signalosome anchored in lipid rafts leads to constitutive activation of NF-kappaB and autonomous cell growth in B cell lymphomas Immunity 2002; 16: 37-50. |
| [13] | Jost PJ, Ruland J. Aberrant NF-kappaB signaling in lymphoma: mechanisms, consequences, and therapeutic implications Blood 2007; 109: 2700-7. |
| [14] | Vallé A, Zuber CE, Defrance T, et al. Activation of human B lymphocytes through CD40 and interleukin 4 Eur J Immunol 1989; 19: 1463-7. |
| [15] | Banchereau J, de Paoli P, Vallé A, et al. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40 Science 1991; 251: 70-2. |
| [16] | Wiesner M, Zentz C, Mayr C, et al. Conditional immortalization of human B cells by CD40 ligation PLoS One 2008 Jan 23; 23: e1464 [about 13 screens]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2180193/ [[cited 2008 January 23]]; |
| [17] | Castillo R, Mascarenhas J, Telford W, et al. Proliferative response of mantle cell lymphoma cells stimulated by CD40 ligation and IL-4 Leukemia 2000; 14: 292-8. |
| [18] | Johnson PW, Watt SM, Betts DR, et al. Isolated follicular lymphoma cells are resistant to apoptosis and can be grown in vitro in the CD40/stromal cell system Blood 1993; 82: 1848-57. |
| [19] | Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray Blood 2004; 103: 275-82. |
| [20] | Schultze JL, Cardoso AA, Freeman GJ, et al. Follicular lymphomas can be induced to present alloantigen efficiently a conceptual model to improve their tumor immunogenicity Proc Natl Acad Sci USA 1995; 92: 8200-4. |
| [21] | Kagami Y, Tobinai K, Kinoshita T, et al. Novel interleukin-2 dependent T-cell line derived from adult T-cell leukemia not associated with human T-cell leukemia virus type I Jpn J Cancer Res 1993; 84: 371-8. |
| [22] | Kagami Y, Jung J, Choi YS, et al. Establishment of a follicular lymphoma cell line (FLK-1) dependent on follicular dendritic cell-like cell line HK Leukemia 2001; 15: 148-56. |
| [23] | Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large-B-cell lymphomas N Engl J Med 2008; 359: 2313-. |
| [24] | Monti S, Savage KJ, Kutok JL, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response Blood 2005; 105: 1851-61. |
| [25] | Paul WE, et al. Interleukin-4 a prototypic immunoregulatory lymphokine Blood 1991; 77: 1859-70. |
| [26] | Nelms K, Huang H, Ryan J, et al. Interleukin-4 receptor signalling mechanisms and their biological significance Adv Exp Med Biol 1998; 452: 37-43. |
| [27] | Taylor CW, Grogan TM, & Salmon SE. Effects of interleukin-4 on the in vitro growth of human lymphoid and plasma cell neoplasms Blood 1990; 75: 1114-8. |
| [28] | Kadoch C, Dinca EB, Voicu R, et al. Pathologic correlates of primary central nervous system lymphoma defined in an orthotopic xenograft model Clin Cancer Res 2009; 15: 1989-97. |
| [29] | Lu X, Nechushtan H, Ding F, et al. Distinct IL-4-induced gene expression, proliferation, and intracellular signaling in germinal center B-cell-like and activated B-cell-like diffuse large-cell lymphomas Blood 2005; 105: 2924-32. |
| [30] | Katzenberger T, Kalla J, Leich E, et al. A distinctive subtype of t(14,18)-negative nodal follicular non-Hodgkin lymphoma characterized by a predominantly diffuse growth pattern and deletions in the chromosomal region 1p36 Blood 2009; 113: 1053-61. |