- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Conference Proceedings Journal
(Biological Sciences, Chemical Sciences, Physical Sciences, Medicine, Engineering & Technology)
(Discontinued)
ISSN: 2210-2892 ― Volume 10, 2020
Activated Salivary MMP-2 - A Potential Breast Cancer Marker
Nabanita Bhattacharyya1, Subhajit Mondal1, Mohammad Nasim Ali1, Ramanuj Mukherjee2, Anjan Adhikari2, Amitava Chatterjee1, *
Abstract
It has been reported that Matrixmetalloproteinase-2 (MMP-2) is involved in the pathogenesis of cancer. The over expression of MMP-2 is associated with the progression of malignancy of several types of carcinoma. Human saliva is a biological fluid with several advantages for non-invasive diagnosis and prognosis of diseases. The aim of this study was to detect MMPs expression and activity in biological fluids (saliva, urine etc.) derived from breast cancer patients. Here, our results showed that the activity of MMP-2 was higher at the time before the surgery than after the saliva collected from the same patients. Therefore, we suggested that the highly active form of MMP-2 presented in saliva could be used as a novel potential biomarker for non-invasive diagnosis of breast cancer.
Article Information
Identifiers and Pagination:
Year: 2017Volume: 8
First Page: 22
Last Page: 32
Publisher Id: TOPROCJ-8-22
DOI: 10.2174/2210289201708010022
Article History:
Received Date: 06/09/2016Revision Received Date: 30/11/2016
Acceptance Date: 12/12/2016
Electronic publication date: 22/03/2017
Collection year: 2017
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Emeritus Scientist, Ramakrishna Mission, Vivekananda University, Narendrapur, Kolkata-700103, India; Tel/Fax: 033-2477-2020; E-mail: amitavachatterjee24@gmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 06-09-2016 |
Original Manuscript | Activated Salivary MMP-2 - A Potential Breast Cancer Marker | |
INTRODUCTION
The matrix metalloproteinases (MMPs), is a family of proteases involved in extracellular matrix remodeling, whose activity has been implicated in a number of important normal and pathologic processes [1Hadler-Olsen, E.; Winberg, J.O.; Uhlin-Hansen, L. Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumour Biol., 2013, 34(4), 2041-2051.
[http://dx.doi.org/10.1007/s13277-013-0842-8] [PMID: 23681802] -5Talvensaari-Mattila, A.; Pääkkö, P.; Turpeenniemi-Hujanen, T. MMP-2 positivity and age less than 40 years increases the risk for recurrence in premenopausal patients with node-positive breast carcinoma. Breast Cancer Res. Treat., 1999, 58(3), 287-293.
[http://dx.doi.org/10.1023/A:1006326513176] [PMID: 10718490] ]. Growth of tumor, progression, and metastasis as well as angiogenesis are associated with these events. As a result, these proteases are important therapeutic and diagnostic targets for detection of human cancers [6Talvensaari-Mattila, A.; Pääkkö, P.; Turpeenniemi-Hujanen, T. Matrix metalloproteinase-2 (MMP-2) is associated with survival in breast carcinoma. Br. J. Cancer, 2003, 89(7), 1270-1275.
[http://dx.doi.org/10.1038/sj.bjc.6601238] [PMID: 14520459] -10Talvensaari-Mattila, A.; Pääkkö, P.; Höyhtyä, M.; Blanco-Sequeiros, G.; Turpeenniemi-Hujanen, T. Matrix metalloproteinase-2 immunoreactive protein: a marker of aggressiveness in breast carcinoma. Cancer, 1998, 83(6), 1153-1162.
[http://dx.doi.org/10.1002/(SICI)1097-0142(19980915)83:6<1153::AID-CNCR14>3.0.CO;2-4] [PMID: 9740080] ]. Successful targeting of this enzyme as diagnostic and prognostic predictors of human cancer has very interesting potential. Matrix metalloproteinase-2 (MMP-2, 72 Kd gelatinase A), a member of zinc dependent endopeptidases, degrades matrix proteins like type IV collagens in basement membranes. The expression of MMP-2 is strongly associated with the progression of malignancy of several types of carcinoma. Presence of active MMP-2 is related to breast cancer development [11Jäälinojä, J.; Herva, R.; Korpela, M.; Höyhtyä, M.; Turpeenniemi-Hujanen, T. Matrix metalloproteinase 2 (MMP-2) immunoreactive protein is associated with poor grade and survival in brain neoplasms. J. Neurooncol., 2000, 46(1), 81-90.
[http://dx.doi.org/10.1023/A:1006421112839] [PMID: 10896208] -15Luukkaa, H.; Klemi, P.; Hirsimäki, P.; Vahlberg, T.; Kivisaari, A.; Kähäri, V.M.; Grénman, R. Matrix metalloproteinase (MMP)-7 in salivary gland cancer. Acta Oncol., 2010, 49(1), 85-90.
[http://dx.doi.org/10.3109/02841860903287197] [PMID: 19929564] ]. The role of MMP in cancer biology especially in migration of malignant tumor cells (metastasis) is well documented [16Das, S.; Banerji, A.; Frei, E.; Chatterjee, A. Rapid expression and activation of MMP-2 and MMP-9 upon exposure of human breast cancer cells (MCF-7) to fibronectin in serum free medium. Life Sci., 2008, 82(9-10), 467-476.
[http://dx.doi.org/10.1016/j.lfs.2007.12.013] [PMID: 18243246] -25Patani, M.R.; Jain, J.; Marya, B.H.; Patel, M.B. Role matrix metalloproteinases 2 in glioma patient specific in age, gender & menopausal status. J. Global Pharm. Technol., 2011, 3(3), 38-44.].
Human saliva is a biological fluid of varying diagnostic potential with several advantages for disease diagnosis and prognosis [26Pai, V.; Kedilaya, H.P.; Vijaykumar, T. Salivary tumor markers: A review. Int. J. Pharm. Chem. Biol. Sci., 2013, 3(3), 510-520.]. Salivary markers have been reported for oral cancer detection [27Stott-Miller, M.; Houck, J.R.; Lohavanichbutr, P.; Méndez, E.; Upton, M.P.; Futran, N.D.; Schwartz, S.M.; Chen, C. Tumor and salivary matrix metalloproteinase levels are strong diagnostic markers of oral squamous cell carcinoma. Cancer Epidemiol. Biomarkers Prev., 2011, 20(12), 2628-2636.
[http://dx.doi.org/10.1158/1055-9965.EPI-11-0503] [PMID: 21960692] ]. A review on salivary proteomics and genomics has been reported [28Brooks, M.N.; Wang, J.; Li, Y.; Zhang, R.; Elashoff, D.; Wong, D.T. Salivary protein factors are elevated in breast cancer patients. Mol. Med. Rep., 2008, 1(3), 375-378.
[PMID: 19844594] ]. In chronic periodontitis the role of MMP-2 and 9 has been observed [29Shah, F.D.; Begum, R.; Vajaria, B.N.; Patel, K.R.; Patel, J.B.; Shukla, S.N.; Patel, P.S. A review on salivary genomics and proteomics biomarkers in oral cancer. Indian J. Clin. Biochem., 2011, 26(4), 326-334.
[http://dx.doi.org/10.1007/s12291-011-0149-8] [PMID: 23024467] ]. It has also been reported that salivary protein factors are elevated in breast cancer patients [26Pai, V.; Kedilaya, H.P.; Vijaykumar, T. Salivary tumor markers: A review. Int. J. Pharm. Chem. Biol. Sci., 2013, 3(3), 510-520.]. Gelatinolytic activity of gingival crevicular fluid has also been observed. While saliva is a source of easily accessible bodily fluid, little efforts have been done to study its potential in cancer diagnosis. In this present project we aimed to develop a non-invasive method to detect MMPs in saliva or urine which may be used for diagnosis and prognosis.
Here we report that MMP-2 is present in a highly active form in saliva of breast cancer patients collected before surgery, however, 7 days after surgery the activity of the MMP-2 decreases as shown in comparative zymogram, indicating potential of salivary MMP-2 as breast cancer marker.
MATERIALS AND METHODS
Patients
Saliva (500 ul) and urine (10 ml) were collected from 36 female breast cancer patients at different stages of the disease (I-IV) before and 7 days after surgery. Saliva from 20 female patients with benign breast tumor was also collected before and 7 days after surgery from R G Kar Medical College, Kolkata following the ethical guidelines. The samples were collected in autoclaved tubes, centrifuged at 5000 rpm for 15 mins at 4°C and clean supernatants were stored (adding protease inhibitors cocktail) at - 20°C until use. Saliva of female breast cancer patients (100 cases) at pre and post surgical conditions (stages I-IV) were also collected, randomly. Saliva of 25 normal (Indian standard, without any disease) female volunteers at ages between 25-50 years were also collected.
Reagents
MMP-2 monoclonal antibody was purchased from Santa Cruz, USA. Protease inhibitor cocktail and NBT/BCIP were purchased from Roche, Germany. All other chemicals like Gelatin, acrylamide, bis-acrylamide, SDS, TRIS etc were purchased from Sigma, USA.
METHODS
Substrate Gel Electrophoresis
Equal amount (100 ug) of proteins from saliva and urine (determined by Lowry's method) run in 8% SDS-PAGE impregnated with 0.1% Gelatin. The gel was run at 15 mA using Tris/Glycine/SDS buffer (pH 8.3). The gel was washed in 2.5% Triton-X for 15 mins and then incubated in buffer A (NaCl 0.2M, CaCl2 4.5 mM, Tris 50mM, pH 7.4) overnight at 37°C. The gel was stained with Coommassie Brilliant Blue to develop the zymogram [16Das, S.; Banerji, A.; Frei, E.; Chatterjee, A. Rapid expression and activation of MMP-2 and MMP-9 upon exposure of human breast cancer cells (MCF-7) to fibronectin in serum free medium. Life Sci., 2008, 82(9-10), 467-476.
[http://dx.doi.org/10.1016/j.lfs.2007.12.013] [PMID: 18243246] ].
Immunoprecipitation
100 ug of salivary proteins (saliva collected before surgery) of breast cancer patient was immunoprecipitated with MMP-2 monoclonal antibody and Protein G-Agarose. A zymogram was developed with the immunoprecipitates.
ELISA
To assay salivary MMP-2, TIMP-2, VEGF 25 ug of salivary proteins were used to develop ELISA using their respective monoclonal antibodies followed by 2nd antibody coupled to horseradish peroxidase (HRP). O.D was taken at 450 nM [16Das, S.; Banerji, A.; Frei, E.; Chatterjee, A. Rapid expression and activation of MMP-2 and MMP-9 upon exposure of human breast cancer cells (MCF-7) to fibronectin in serum free medium. Life Sci., 2008, 82(9-10), 467-476.
[http://dx.doi.org/10.1016/j.lfs.2007.12.013] [PMID: 18243246] ].
Immunoblot Development
100 ug of salivary proteins were run on 8% SDS-PAGE. Proteins were transferred onto nitrocellulose membrane and immunoblots were developed using monoclonal antibody against MMP-2 (Santa Cruz, USA) followed by alkaline phosphatase coupled 2nd antibody. The color was developed using NBT/BCIP [16Das, S.; Banerji, A.; Frei, E.; Chatterjee, A. Rapid expression and activation of MMP-2 and MMP-9 upon exposure of human breast cancer cells (MCF-7) to fibronectin in serum free medium. Life Sci., 2008, 82(9-10), 467-476.
[http://dx.doi.org/10.1016/j.lfs.2007.12.013] [PMID: 18243246] ].
Statistical Analysis
Statistical Analysis was performed with help of Epi Info (TM) 3.5.3. EPI INFO is a trademark of the Centers for Disease Control and Prevention (CDC). Descriptive statistical analysis was performed to calculate the means with corresponding standard deviations (s.d.). Test of proportion was used to find the Standard Normal Deviate (Z) to compare the difference proportions. t-test was used to compare the means. p <0.05 was taken to be statistically significant.
RESULTS
Expression and Activity of MMP-2 in Saliva & Urine of Breast Cancer Patients
Fig. (1A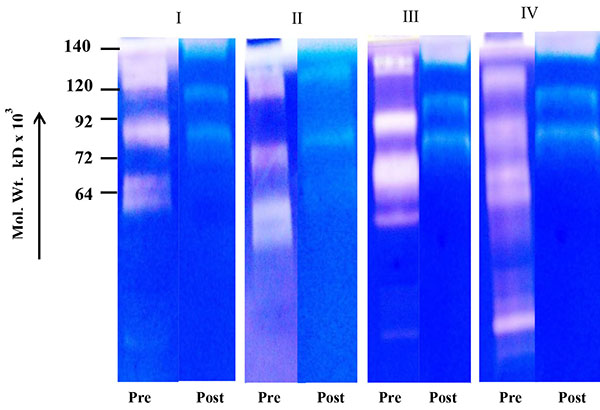 ) shows comparative zymogram of salivary MMPs of breast cancer patients (before and after surgery of the same patient) at different stages of the disease (I-IV). The results show higher activity of MMP-2 (increasing stage wise) in saliva of breast cancer patients collected before surgery (72 kD pro MMP-2 when activated broken down to smaller fragments). The activity of MMP-2 is at background level in post-operative saliva of the same breast cancer patients. Fig. (1B
) shows comparative zymogram of salivary MMPs of breast cancer patients (before and after surgery of the same patient) at different stages of the disease (I-IV). The results show higher activity of MMP-2 (increasing stage wise) in saliva of breast cancer patients collected before surgery (72 kD pro MMP-2 when activated broken down to smaller fragments). The activity of MMP-2 is at background level in post-operative saliva of the same breast cancer patients. Fig. (1B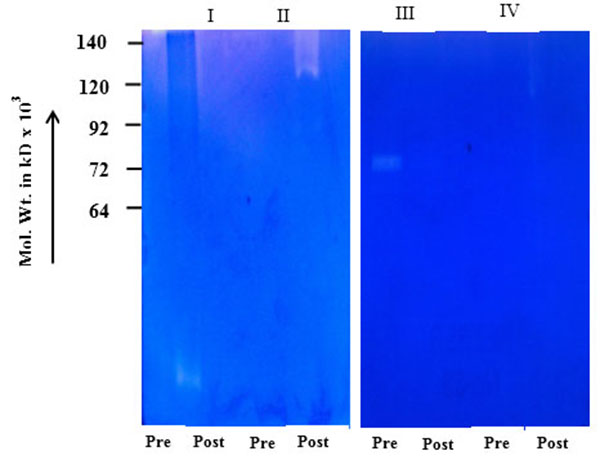 ) shows that urine samples (collected before and after surgery) of the same breast cancer patients (of Fig. 1A
) shows that urine samples (collected before and after surgery) of the same breast cancer patients (of Fig. 1A ) express very weak background of MMP activity (for example saliva of one breast cancer patient at stage IV (before surgery) is showing MMP activity from 140 Kd to 25 Kd in comparison with the same patient's urine showing background activity). Fig. (1C
) express very weak background of MMP activity (for example saliva of one breast cancer patient at stage IV (before surgery) is showing MMP activity from 140 Kd to 25 Kd in comparison with the same patient's urine showing background activity). Fig. (1C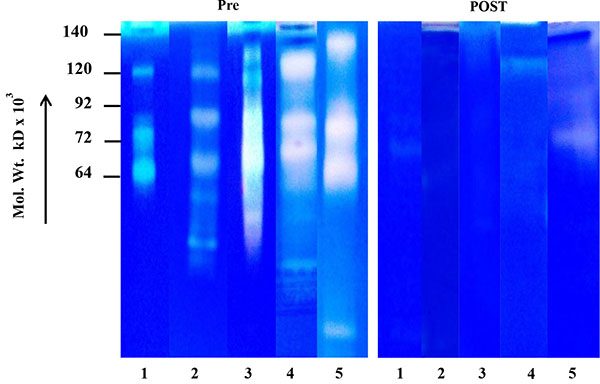 ) shows the salivary MMP-2 activity of the breast cancer patients randomly collected before and after surgery. The comparative zymogram shows higher MMP-2 activity (from stages I-IV) as compared to background activity in saliva collected after surgery. Fig. (1D
) shows the salivary MMP-2 activity of the breast cancer patients randomly collected before and after surgery. The comparative zymogram shows higher MMP-2 activity (from stages I-IV) as compared to background activity in saliva collected after surgery. Fig. (1D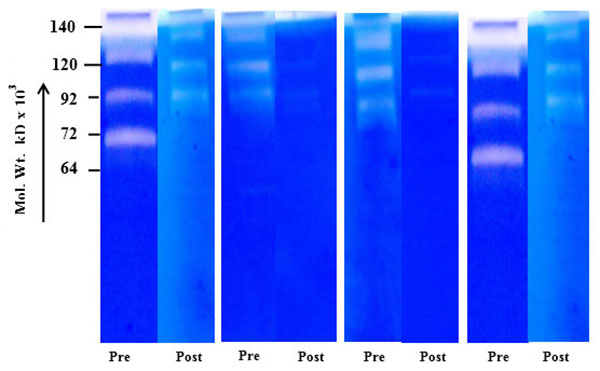 ) shows benign breast tumors, comparative zymogram (saliva collected before and after surgery) shows background activity at MMP-2 region (pro or activated) and Fig. (1E
) shows benign breast tumors, comparative zymogram (saliva collected before and after surgery) shows background activity at MMP-2 region (pro or activated) and Fig. (1E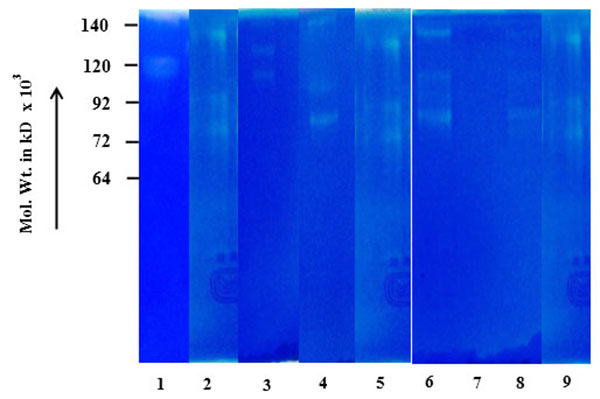 ) shows background MMP activity in case of normal female volunteers.
) shows background MMP activity in case of normal female volunteers.
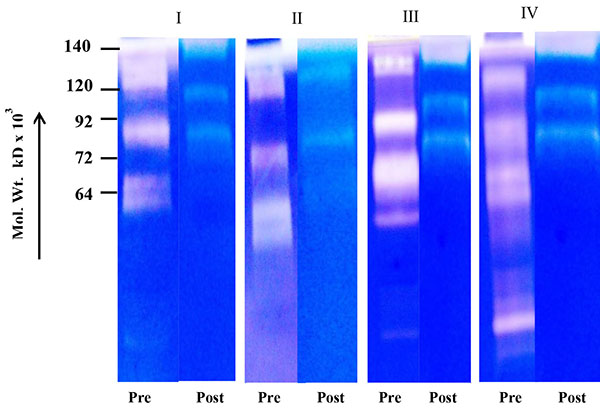 |
Fig. (1A) before and after surgery of stages I-IV. |
 |
Fig. (1B) Urinary MMP-2 profile of breast cancer Patients (same) before and after surgery of stages I-IV. |
 |
Fig. (1C) Salivary MMP-2 profile of breast cancer patients (random) before(pre stagewise) and after(post) surgery. |
 |
Fig. (1D) Salivary MMP-2 profile of breast tumor (benign) patients before and after surgery. |
 |
Fig. (1E) Salivary MMP-2 profile in normal femalevolunteers. |
Immunoblot of Saliva Sample of Breast Cancer Patients
To identify MMP species immunoblot of salivary proteins was developed using MMP-2 monoclonal antibody which shows pro (72 kD) and activated MMP-2 (at 64 kD, 50 kD etc.) and 140 kD higher molecular weight form in the salivary sample of the breast cancer patients (collected before surgery) very clearly (Fig. 2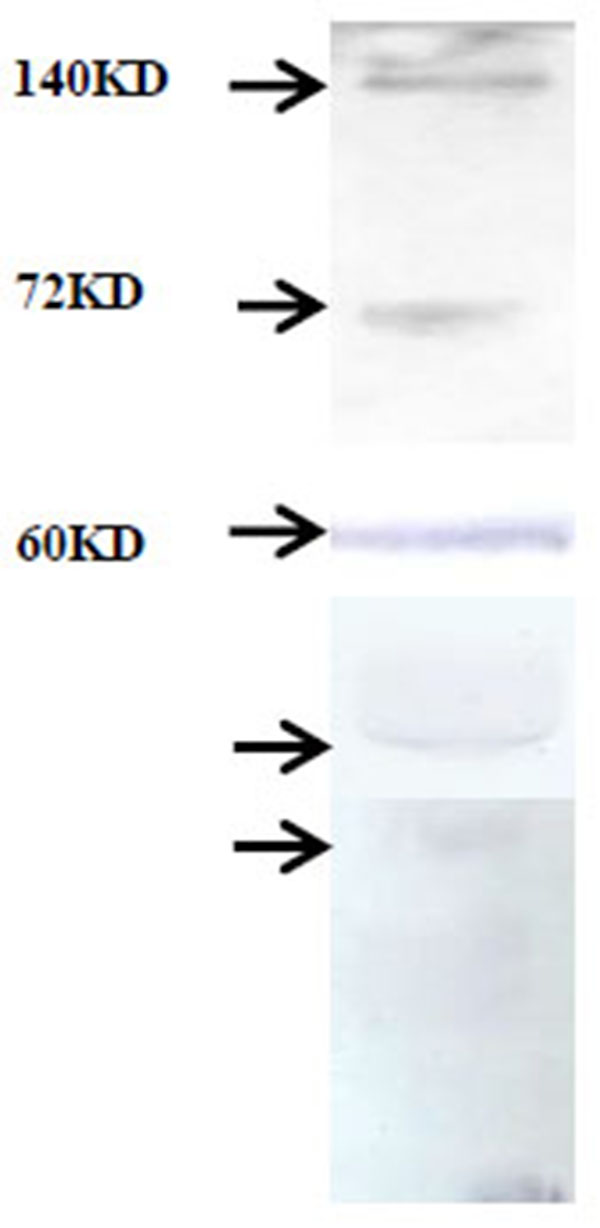 ).
).
 |
Fig. (2) Immunoblot of breast cancer patient salivary proteins with MMP-2 monoclonal antibody. |
Immunoprecipitation
Fig. (3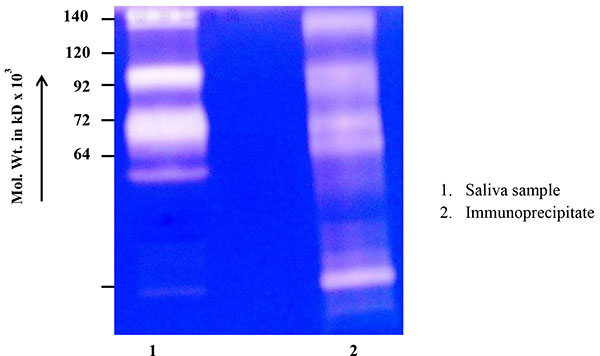 ) shows the immunoprecipitation of salivary proteins with MMP-2 monoclonal antibody. The zymogram clearly demonstrates that most of the higher and the lower molecular bands have been precipitated by MMP-2 antibody, confirming the MMP-2 species.
) shows the immunoprecipitation of salivary proteins with MMP-2 monoclonal antibody. The zymogram clearly demonstrates that most of the higher and the lower molecular bands have been precipitated by MMP-2 antibody, confirming the MMP-2 species.
 |
Fig. (3) Immunoprecipitation of salivary proteins with MMP- 2 monoclonal antibody. |
ELISA
The quantitation of MMP-2, TIMP-2, and VEGF was performed in 10 saliva samples, collected at pre and post surgery Figs. (4A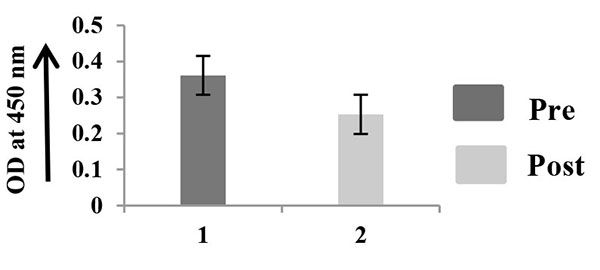 -4C
-4C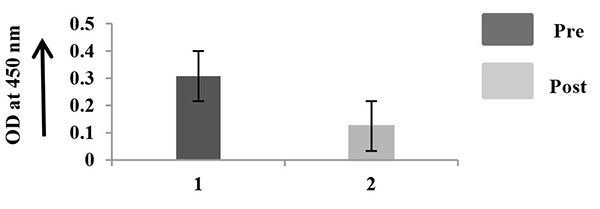 ). This procedure was done in triplicate. The comparative ELISA clearly shows that MMP-2 is comparatively little higher in saliva of pre surgical samples than that of post surgical samples (Fig. 4A
). This procedure was done in triplicate. The comparative ELISA clearly shows that MMP-2 is comparatively little higher in saliva of pre surgical samples than that of post surgical samples (Fig. 4A ). TIMP-2 is appreciably higher in saliva collected after surgery (Fig. 4B
). TIMP-2 is appreciably higher in saliva collected after surgery (Fig. 4B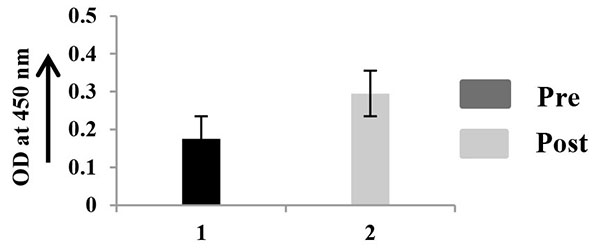 ) and VEGF (Fig. 4C
) and VEGF (Fig. 4C ) is higher in saliva collected before surgery than after surgery, p < 0.05.
) is higher in saliva collected before surgery than after surgery, p < 0.05.
 |
Fig. (4A) ELISA of salivary MMP-2. |
 |
Fig. (4B) ELISA of salivary TIMP-2. |
 |
Fig. (4C) ELISA of salivary VEGF. |
Clinical Characteristics of the Study Population
The mean age (mean ± s.d.) of the patients was 48.52±10.90 years with range 18-70 years and the median age was 49.5 years. Proportion of patients in the age group 30 - 69 years (94.4%) was significantly higher than other age group (Z= 12.95; p <0.0001).
Fifty eight point eight percent (58.8%) of the cases were with ER positive and the rest 41.2% were ER negative cases. 53.0% of the cases were with PR positive and rest of the 41.2% were PR negative cases. 27.3% of the cases were with Her2 neu positive and rest of the 72.7% were Her2 neu negative cases. 14 out of 34 cases (41.1%) were TNBC (triple negative breast cancer).
DISCUSSION
In this present communication, we report the identification of activated MMP-2 (with increase in activity from early to late stage) in saliva of breast cancer patients collected before surgery. Interestingly, saliva collected after surgery (within 7 days) from the same patients, showed very weak background MMP-2 activity. The urine of the same breast cancer patients does not show any appreciable MMP activity in pre or post surgical samples. Expression of urinary MMPs has been reported by other groups in other type of cancer patients [30Maquoi, E.; Assent, D.; Detilleux, J.; Pequeux, C.; Foidart, J.M.; Noël, A. MT1-MMP protects breast carcinoma cells against type I collagen-induced apoptosis. Oncogene, 2012, 31(4), 480-493.
[http://dx.doi.org/10.1038/onc.2011.249] [PMID: 21706048] -33Sier, C.F.; Casetta, G.; Verheijen, J.H.; Tizzani, A.; Agape, V.; Kos, J.; Blasi, F.; Hanemaaijer, R. Enhanced urinary gelatinase activities (matrix metalloproteinases 2 and 9) are associated with early-stage bladder carcinoma: a comparison with clinically used tumor markers. Clin. Cancer Res., 2000, 6(6), 2333-2340.
[PMID: 10873084] ]. The immunoblot shows the presence of pro (72 kD), activated (64,50 kD etc.) MMP-2. A band at 140 kD region is also visible clearly indicating that MMP-2 may be complexed with other protein. The immune precipitation of saliva with MMP-2 antibody may strongly indicate that the salivary MMPs are mainly MMP-2 pro and activated products with higher molecular weight forms. Interestingly, out of 36 patients 35 patients are ductal carcimona only one patient is invasive lobular carcinoma but with similar results.
VEGF has been reported to be an important regulator of MMP-2 activity [34Masood, R.; Cai, J.; Zheng, T.; Smith, D.L.; Hinton, D.R.; Gill, P.S. Vascular endothelial growth factor (VEGF) is an autocrine growth factor for VEGF receptor-positive human tumors. Blood, 2001, 98(6), 1904-1913.
[http://dx.doi.org/10.1182/blood.V98.6.1904] [PMID: 11535528] -38Toi, M.; Matsumoto, T.; Bando, H. Vascular endothelial growth factor: its prognostic, predictive, and therapeutic implications. Lancet Oncol., 2001, 2(11), 667-673.
[http://dx.doi.org/10.1016/S1470-2045(01)00556-3] [PMID: 11902537] ]. The VEGF concentration of saliva of pre and post-surgical samples were measured and was found to be appreciably higher in pre surgical saliva as compared to post-surgical saliva. The TIMP-2 reported to be MMP-2 inhibitor [39Rodrigues, M.; Xin, X.; Jee, K.; Babapoor-Farrokhran, S.; Kashiwabuchi, F.; Ma, T.; Bhutto, I.; Hassan, S.J.; Daoud, Y.; Baranano, D.; Solomon, S.; Lutty, G.; Semenza, G.L.; Montaner, S.; Sodhi, A. VEGF secreted by hypoxic Müller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes, 2013, 62(11), 3863-3873.
[http://dx.doi.org/10.2337/db13-0014] [PMID: 23884892] -43Giannelli, G.; Bergamini, C.; Marinosci, F.; Fransvea, E.; Quaranta, M.; Lupo, L.; Schiraldi, O.; Antonaci, S. Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular carcinoma. Int. J. Cancer, 2002, 97(4), 425-431.
[http://dx.doi.org/10.1002/ijc.1635] [PMID: 11802202] ] was found to be higher in post-surgical saliva as compared to pre surgical saliva. The higher concentration of TIMP-2 in saliva collected 7 days after surgery and higher VEGF concentration of saliva collected before surgery highlight the higher MMP-2 activity in saliva of breast cancer patients collected before surgery. Table 5 shows the comparative picture of salivary MMP-2 activity in before and after surgery (same patient) and ER/PR/Her-2 Neu status of 36 breast cancer female patients at different stages of the disease.
Descriptive statistical analysis was performed and reported in Tables 1-6. In summary, we propose that saliva is a novel biological fluid to detect highly activated MMP-2, a very important MMP species in the development of cancer. Our observation that the expression and activity of salivary MMP-2 is appreciably high in saliva of breast cancer patients (stagewise I-IV) collected before surgery as compared to salivary MMP-2 collected 7 days after surgery of the same breast cancer patients is a novel finding. This salivary MMP-2 activity may be translated to potential breast cancer marker for diagnosis which is reproducible, cost effective and a non-invasive method for breast cancer detection.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors wish to acknowledge Vice Chancellor, Ramakrishna Mission Vivekananda University for providing infrastructural facilities and Department of Science and Technology, Govt of West Bengal for financial support (Sanction No. 528(Sanc)/ST/P/S&T/9G-3/2014).
REFERENCES
| [1] | Hadler-Olsen, E.; Winberg, J.O.; Uhlin-Hansen, L. Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumour Biol., 2013, 34(4), 2041-2051. [http://dx.doi.org/10.1007/s13277-013-0842-8] [PMID: 23681802] |
| [2] | Li, H.C.; Cao, D.C.; Liu, Y.; Hou, Y.F.; Wu, J.; Lu, J.S.; Di, G.H.; Liu, G.; Li, F.M.; Ou, Z.L.; Jie, C.; Shen, Z.Z.; Shao, Z.M. Prognostic value of matrix metalloproteinases (MMP-2 and MMP-9) in patients with lymph node-negative breast carcinoma. Breast Cancer Res. Treat., 2004, 88(1), 75-85. [http://dx.doi.org/10.1007/s10549-004-1200-8] [PMID: 15538048] |
| [3] | Brown, P.D.; Bloxidge, R.E.; Anderson, E.; Howell, A. Expression of activated gelatinase in human invasive breast carcinoma. Clin. Exp. Metastasis, 1993, 11(2), 183-189. [http://dx.doi.org/10.1007/BF00114976] [PMID: 8444010] |
| [4] | Sullu, Y.; Sullu, Y.; Demirag, G G.; Yildirim, A.; Karagoz, F.; Kandemir, B. Matrix metalloproteinase-2 (MMP-2) and MMP-9 expression in invasive ductal carcinoma of the breast. Pathol. - Res. Pract., 2011, 207, 747-753. |
| [5] | Talvensaari-Mattila, A.; Pääkkö, P.; Turpeenniemi-Hujanen, T. MMP-2 positivity and age less than 40 years increases the risk for recurrence in premenopausal patients with node-positive breast carcinoma. Breast Cancer Res. Treat., 1999, 58(3), 287-293. [http://dx.doi.org/10.1023/A:1006326513176] [PMID: 10718490] |
| [6] | Talvensaari-Mattila, A.; Pääkkö, P.; Turpeenniemi-Hujanen, T. Matrix metalloproteinase-2 (MMP-2) is associated with survival in breast carcinoma. Br. J. Cancer, 2003, 89(7), 1270-1275. [http://dx.doi.org/10.1038/sj.bjc.6601238] [PMID: 14520459] |
| [7] | Talvensaari-Mattila, A.; Pääkko, P.; Blanco-Sequeiros, G.; Turpeenniemi-Hujanen, T. Matrix metalloproteinase-2 (MMP-2) is associated with the risk for a relapse in postmenopausal patients with node-positive breast carcinoma treated with antiestrogen adjuvant therapy. Breast Cancer Res. Treat., 2001, 65(1), 55-61. [http://dx.doi.org/10.1023/A:1006458601568] [PMID: 11245340] |
| [8] | Tryggvason, K.; Höyhtyä, M.; Pyke, C. Type IV collagenases in invasive tumors. Breast Cancer Res. Treat., 1993, 24(3), 209-218. [http://dx.doi.org/10.1007/BF01833261] [PMID: 8435476] |
| [9] | Väisänen, A.; Kallioinen, M.; Taskinen, P.J.; Turpeenniemi-Hujanen, T. Prognostic value of MMP-2 immunoreactive protein (72 kD type IV collagenase) in primary skin melanoma. J. Pathol., 1998, 186(1), 51-58. [http://dx.doi.org/10.1002/(SICI)1096-9896(199809)186:1<51::AID-PATH131>3.0.CO;2-P] [PMID: 9875140] |
| [10] | Talvensaari-Mattila, A.; Pääkkö, P.; Höyhtyä, M.; Blanco-Sequeiros, G.; Turpeenniemi-Hujanen, T. Matrix metalloproteinase-2 immunoreactive protein: a marker of aggressiveness in breast carcinoma. Cancer, 1998, 83(6), 1153-1162. [http://dx.doi.org/10.1002/(SICI)1097-0142(19980915)83:6<1153::AID-CNCR14>3.0.CO;2-4] [PMID: 9740080] |
| [11] | Jäälinojä, J.; Herva, R.; Korpela, M.; Höyhtyä, M.; Turpeenniemi-Hujanen, T. Matrix metalloproteinase 2 (MMP-2) immunoreactive protein is associated with poor grade and survival in brain neoplasms. J. Neurooncol., 2000, 46(1), 81-90. [http://dx.doi.org/10.1023/A:1006421112839] [PMID: 10896208] |
| [12] | Chambers, A.F.; Matrisian, L.M. Changing views of the role of matrix metalloproteinases in metastasis. J. Natl. Cancer Inst., 1997, 89(17), 1260-1270. [http://dx.doi.org/10.1093/jnci/89.17.1260] [PMID: 9293916] |
| [13] | Curran, S.; Murray, G.I. Matrix metalloproteinases in tumour invasion and metastasis. J. Pathol., 1999, 189(3), 300-308. [http://dx.doi.org/10.1002/(SICI)1096-9896(199911)189:3<300::AID-PATH456>3.0.CO;2-C] [PMID: 10547590] |
| [14] | Koujan, S.E.; Gargarib, B.P.; Pirouzpanah, S.; Khalili, M. Matrix Metalloproteinases and Breast Cancer. Thrita, 2015, 4(1), e21959. |
| [15] | Luukkaa, H.; Klemi, P.; Hirsimäki, P.; Vahlberg, T.; Kivisaari, A.; Kähäri, V.M.; Grénman, R. Matrix metalloproteinase (MMP)-7 in salivary gland cancer. Acta Oncol., 2010, 49(1), 85-90. [http://dx.doi.org/10.3109/02841860903287197] [PMID: 19929564] |
| [16] | Das, S.; Banerji, A.; Frei, E.; Chatterjee, A. Rapid expression and activation of MMP-2 and MMP-9 upon exposure of human breast cancer cells (MCF-7) to fibronectin in serum free medium. Life Sci., 2008, 82(9-10), 467-476. [http://dx.doi.org/10.1016/j.lfs.2007.12.013] [PMID: 18243246] |
| [17] | Strongin, A.Y.; Collier, I.; Bannikov, G.; Marmer, B.L.; Grant, G.A.; Goldberg, G.I. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J. Biol. Chem., 1995, 270(10), 5331-5338. [http://dx.doi.org/10.1074/jbc.270.10.5331] [PMID: 7890645] |
| [18] | Sameshima, T.; Nabeshima, K.; Toole, B.P.; Yokogami, K.; Okada, Y.; Goya, T.; Koono, M.; Wakisaka, S. Glioma cell extracellular matrix metalloproteinase inducer (EMMPRIN) (CD147) stimulates production of membrane-type matrix metalloproteinases and activated gelatinase A in co-cultures with brain-derived fibroblasts. Cancer Lett., 2000, 157(2), 177-184. [http://dx.doi.org/10.1016/S0304-3835(00)00485-7] [PMID: 10936678] |
| [19] | Polette, M.; Nawrocki, B.; Gilles, C.; Sato, H.; Seiki, M.; Tournier, J.M.; Birembaut, P. MT-MMP expression and localisation in human lung and breast cancers. Virchows Arch., 1996, 428(1), 29-35. [http://dx.doi.org/10.1007/BF00192924] [PMID: 8646366] |
| [20] | Sato, H.; Takino, T.; Okada, Y.; Cao, J.; Shinagawa, A.; Yamamoto, E.; Seiki, M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature, 1994, 370(6484), 61-65. [http://dx.doi.org/10.1038/370061a0] [PMID: 8015608] |
| [21] | Ellenrieder, V.; Alber, B.; Lacher, U.; Hendler, S.F.; Menke, A.; Boeck, W.; Wagner, M.; Wilda, M.; Friess, H.; Büchler, M.; Adler, G.; Gress, T.M. Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int. J. Cancer, 2000, 85(1), 14-20. [http://dx.doi.org/10.1002/(SICI)1097-0215(20000101)85:1<14::AID-IJC3>3.0.CO;2-O] [PMID: 10585576] |
| [22] | Sillanpää, S.; Anttila, M.; Suhonen, K.; Hämäläinen, K.; Turpeenniemi-Hujanen, T.; Puistola, U.; Tammi, M.; Sironen, R.; Saarikoski, S.; Kosma, V.M. Prognostic significance of extracellular matrix metalloproteinase inducer and matrix metalloproteinase 2 in epithelial ovarian cancer. Tumour Biol., 2007, 28(5), 280-289. [http://dx.doi.org/10.1159/000110426] [PMID: 17962725] |
| [23] | Sil, H.; Moulik, S.; Singha, I.; Mandal, S.S.; Biswas, J.; Chatterjee, A. Activation of MMP-2 in Breast cancer Progression. Jour Tumor, 2015, 18 3(1), 292-304. |
| [24] | Roy, R.; Yang, J.; Moses, M.A. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J. Clin. Oncol., 2009, 27(31), 5287-5297. [http://dx.doi.org/10.1200/JCO.2009.23.5556] [PMID: 19738110] |
| [25] | Patani, M.R.; Jain, J.; Marya, B.H.; Patel, M.B. Role matrix metalloproteinases 2 in glioma patient specific in age, gender & menopausal status. J. Global Pharm. Technol., 2011, 3(3), 38-44. |
| [26] | Pai, V.; Kedilaya, H.P.; Vijaykumar, T. Salivary tumor markers: A review. Int. J. Pharm. Chem. Biol. Sci., 2013, 3(3), 510-520. |
| [27] | Stott-Miller, M.; Houck, J.R.; Lohavanichbutr, P.; Méndez, E.; Upton, M.P.; Futran, N.D.; Schwartz, S.M.; Chen, C. Tumor and salivary matrix metalloproteinase levels are strong diagnostic markers of oral squamous cell carcinoma. Cancer Epidemiol. Biomarkers Prev., 2011, 20(12), 2628-2636. [http://dx.doi.org/10.1158/1055-9965.EPI-11-0503] [PMID: 21960692] |
| [28] | Brooks, M.N.; Wang, J.; Li, Y.; Zhang, R.; Elashoff, D.; Wong, D.T. Salivary protein factors are elevated in breast cancer patients. Mol. Med. Rep., 2008, 1(3), 375-378. [PMID: 19844594] |
| [29] | Shah, F.D.; Begum, R.; Vajaria, B.N.; Patel, K.R.; Patel, J.B.; Shukla, S.N.; Patel, P.S. A review on salivary genomics and proteomics biomarkers in oral cancer. Indian J. Clin. Biochem., 2011, 26(4), 326-334. [http://dx.doi.org/10.1007/s12291-011-0149-8] [PMID: 23024467] |
| [30] | Maquoi, E.; Assent, D.; Detilleux, J.; Pequeux, C.; Foidart, J.M.; Noël, A. MT1-MMP protects breast carcinoma cells against type I collagen-induced apoptosis. Oncogene, 2012, 31(4), 480-493. [http://dx.doi.org/10.1038/onc.2011.249] [PMID: 21706048] |
| [31] | Coticchia, C.M.; Curatolo, A.S.; Zurakowski, D.; Yang, J.; Daniels, K.E.; Matulonis, U.A.; Moses, M.A. Urinary MMP-2 and MMP-9 predict the presence of ovarian cancer in women with normal CA125 levels. Gynecol. Oncol., 2011, 123(2), 295-300. [http://dx.doi.org/10.1016/j.ygyno.2011.07.034] [PMID: 21889192] |
| [32] | Roy, R.; Louis, G.; Loughlin, K.R.; Wiederschain, D.; Kilroy, S.M.; Lamb, C.C.; Zurakowski, D.; Moses, M.A. Tumor-specific urinary matrix metalloproteinase fingerprinting: identification of high molecular weight urinary matrix metalloproteinase species. Clin. Cancer Res., 2008, 14(20), 6610-6617. [http://dx.doi.org/10.1158/1078-0432.CCR-08-1136] [PMID: 18927302] |
| [33] | Sier, C.F.; Casetta, G.; Verheijen, J.H.; Tizzani, A.; Agape, V.; Kos, J.; Blasi, F.; Hanemaaijer, R. Enhanced urinary gelatinase activities (matrix metalloproteinases 2 and 9) are associated with early-stage bladder carcinoma: a comparison with clinically used tumor markers. Clin. Cancer Res., 2000, 6(6), 2333-2340. [PMID: 10873084] |
| [34] | Masood, R.; Cai, J.; Zheng, T.; Smith, D.L.; Hinton, D.R.; Gill, P.S. Vascular endothelial growth factor (VEGF) is an autocrine growth factor for VEGF receptor-positive human tumors. Blood, 2001, 98(6), 1904-1913. [http://dx.doi.org/10.1182/blood.V98.6.1904] [PMID: 11535528] |
| [35] | Ferrara, N. Vascular endothelial growth factor: basic science and clinical progress. Endocr. Rev., 2004, 25(4), 581-611. [http://dx.doi.org/10.1210/er.2003-0027] [PMID: 15294883] |
| [36] | Linderholm, B.; Tavelin, B.; Grankvist, K.; Henriksson, R. Vascular endothelial growth factor is of high prognostic value in node-negative breast carcinoma. J. Clin. Oncol., 1998, 16(9), 3121-3128. [PMID: 9738584] |
| [37] | Margolin, K. Inhibition of vascular endothelial growth factor in the treatment of solid tumors. Curr. Oncol. Rep., 2002, 4(1), 20-28. [http://dx.doi.org/10.1007/s11912-002-0044-9] [PMID: 11734110] |
| [38] | Toi, M.; Matsumoto, T.; Bando, H. Vascular endothelial growth factor: its prognostic, predictive, and therapeutic implications. Lancet Oncol., 2001, 2(11), 667-673. [http://dx.doi.org/10.1016/S1470-2045(01)00556-3] [PMID: 11902537] |
| [39] | Rodrigues, M.; Xin, X.; Jee, K.; Babapoor-Farrokhran, S.; Kashiwabuchi, F.; Ma, T.; Bhutto, I.; Hassan, S.J.; Daoud, Y.; Baranano, D.; Solomon, S.; Lutty, G.; Semenza, G.L.; Montaner, S.; Sodhi, A. VEGF secreted by hypoxic Müller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes, 2013, 62(11), 3863-3873. [http://dx.doi.org/10.2337/db13-0014] [PMID: 23884892] |
| [40] | Strongin, A.Y.; Marmer, B.L.; Grant, G.A.; Goldberg, G.I. Plasma membrane-dependent activation of the 72-kDa type IV collagenase is prevented by complex formation with TIMP-2. J. Biol. Chem., 1993, 268(19), 14033-14039. [PMID: 8314771] |
| [41] | Sakata, K.; Shigemasa, K.; Nagai, N.; Ohama, K. Expression of matrix metalloproteinases (MMP-2, MMP-9, MT1-MMP) and their inhibitors (TIMP-1, TIMP-2) in common epithelial tumors of the ovary. Int. J. Oncol., 2000, 17(4), 673-681. [PMID: 10995877] |
| [42] | Łukaszewicz-Zając, M.; Mroczko, B.; Guzińska-Ustymowicz, K.; Pryczynicz, A.; Gryko, M.; Kemona, A.; Kędra, B.; Szmitkowski, M. MMP-2 and their tissue inhibits (TIMP-2) in gastric cancer patients. Adv. Med. Sci., 2013, V(58)(Issue 2), 235-243. |
| [43] | Giannelli, G.; Bergamini, C.; Marinosci, F.; Fransvea, E.; Quaranta, M.; Lupo, L.; Schiraldi, O.; Antonaci, S. Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular carcinoma. Int. J. Cancer, 2002, 97(4), 425-431. [http://dx.doi.org/10.1002/ijc.1635] [PMID: 11802202] |