- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Waste Management Journal
(Discontinued)
ISSN: 1876-4002 ― Volume 13, 2020
Alkalic Leaching and Stabilization of Arsenic from Pyrite Cinders
Yongliang Wang1, Li Xiao1, 2, Ya Liu1, 2, Guoyan Fu1, 2, Shufeng Ye1, *, Yunfa Chen1
Abstract
Introduction:
Pyrite cinder is one of the important secondary resources, but typically contains a certain amount of arsenic, which is harmful to metallurgical process. It usually hopes to remove the arsenic prior to recycle the valuable element in the pyrite cinders.
Methods & Materials:
In this study, the arsenic in the cinders was selectively removed using the alkalic leaching method so as to reduce the loss of ferric and other valuable elements.
Results & Discussion:
The content of arsenic in pyrite cinders was reduced to 0.08% through the investigation of the factors, including particle size, alkaline concentration, temperature, solid-liquid ratio (S/L) and leaching time. Then, the ferric precipitation method was used to remove the arsenic in the leaching solution. More than 99% of the arsenic can be removed by controlling the pH and the ratio of ferric and arsenic (Fe/As) in ambient temperature, and the arsenic concentration in the solution was reduced to less than 0.5mg/L.
Conclusion:
It was found that the precipitated arsenic was mainly amorphous based on the analysis of sediment.
Article Information
Identifiers and Pagination:
Year: 2017Volume: 10
First Page: 41
Last Page: 50
Publisher Id: TOWMJ-10-41
DOI: 10.2174/1876400201710010041
Article History:
Received Date: 31/08/2017Revision Received Date: 2/11/2017
Acceptance Date: 17/11/2017
Electronic publication date: 30/11/2017
Collection year: 2017
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Key Lab. Multi-phase Complex System, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China; Tel: 010-82544923; E-mail: sfye@ipe.ac.cn
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 31-08-2017 |
Original Manuscript | Alkalic Leaching and Stabilization of Arsenic from Pyrite Cinders | |
1. INTRODUCTION
Pyrite cinders are the waste residues generated during the process of producing sulfuric acid from pyrite, and 0.7-1.0t pyrite cinders will be generated in order to obtain 1.0t sulfuric acid. In China, more than 30% sulfuric acid is produced from pyrites, and at least 10 million tons of pyrite cinders are produced through the roasting of pyrite [1Z. Liu, and Y. Zheng, "Effect of Fe(II) on the formation of iron oxide synthesized from pyrite cinders by hydrothermal process", Powder Technol., vol. 209, pp. 119-123.
[http://dx.doi.org/10.1016/j.powtec.2011.02.019] ]. However, only 30% pyrite cinders are recycled as the building materials such as cement, brick and roof tiles, while most of the remaining cinders are dumped as waste residues [2A.V. Abdrakhimov, E.S. Abdrakhimova, and V.Z. Abdrakhimov, "Technical properties of roof tiles made of technogenic material with pyrite cinder", Glass Ceram., vol. 63, no. 3, pp. 130-132.
[http://dx.doi.org/10.1007/s10717-006-0058-0] , 3I. Alp, H. Deveci, E.Y. Yazc, T. Türk, and Y. H. Süngün, "Süngün, Potential use of pyrite cinders as raw material in cement production: Results of industrial scale trial operations", J. Hazard. Mater., vol. 166, no. 1, pp. 144-149.]. This is not only occupying a large number of lands, but also may cause serious environmental pollution to the soil, water and air around the storage ground, which may endanger the public health and safety [4G. Fellet, L. Marchiol, D. Perosab, and G. Zerbi, "The application of phytoremediation technology in a soil contaminated by pyrite cinders", Ecol. Eng., vol. 31, pp. 207-214.
[http://dx.doi.org/10.1016/j.ecoleng.2007.06.011] ].
With the continuous utilization of mineral resources, recycling of the valuable metals from pyrite cinders has received more and more attention, which is also beneficial to the environmental protection. The main component of the cinders is hematite (Fe2O3), and generally contains 20-60% ferric [5Y. Zheng, and Z. Liu, "Preparation of monodispersed mica—teeous iron oxide pigment from pyrite cinders", Powder Technol., vol. 207, pp. 335-342.
[http://dx.doi.org/10.1016/j.powtec.2010.11.015] ], in which the high iron slag has a greater value of recycling. The iron oxide concentrate can be extracted through beneficiation, pelletization and other processes, and used as raw material for blast furnace [6B. He, X. Tian, Y. Sun, C. Yang, Y. Zeng, Y. Wang, S. Zhang, and Z. Pi, "Recovery of iron oxide concentrate from high-sulfur and low-grade pyrite cinder using an innovative beneficiating process", Hydrometallurgy, vol. 104, pp. 241-246.
[http://dx.doi.org/10.1016/j.hydromet.2010.06.009] , 7N. Tugrul, E.M. Derun, and M. Piskin, "Utilization of pyrite ash wastes by pelletization process", Powder Technol., vol. 176, no. 2, pp. 72-76.
[http://dx.doi.org/10.1016/j.powtec.2007.01.012] ]. In addition, the pyrite cinders can also be used to prepare iron-based pigments, iron oxide and so on [5Y. Zheng, and Z. Liu, "Preparation of monodispersed mica—teeous iron oxide pigment from pyrite cinders", Powder Technol., vol. 207, pp. 335-342.
[http://dx.doi.org/10.1016/j.powtec.2010.11.015] , 8Z. Zou, A. Xuan, Z. Yan, Y. Wu, and N. Li, "Preparation of Fe304 particles from copper/iron Ore cinder and their microwave absorption properties", Chem. Eng. Sci., vol. 65, no. 1, pp. 160-164.
[http://dx.doi.org/10.1016/j.ces.2009.06.003] ], or direct reduction of the cinders to obtain metals [9X. Xing, J. Zhang, Z. Wang, K. Jiao, X. Liu, and S. Ren, "Reduction of pyrite cinder pellets mixed with coal powder", J. Iron Steel Res. Int., vol. 21, no. 7, pp. 653-659.
[http://dx.doi.org/10.1016/S1006-706X(14)60101-1] ]. The cinders also contain a small amount of Cu, Zn, Pb, Au, Ag and other valuable metals, which are important for recycling [10Z. Lin, and Q. Ulf, "Predicting the mobility of Zn, Fe, Cu, Pb, Cd from roasted sulfide (pyrite) residues-a case study of wastes from the sulfuric acid industry in Sweden", Waste Manag., vol. 16, no. 8, pp. 671-681.
[http://dx.doi.org/10.1016/S0956-053X(97)00009-3] , 11C. Yang, Y. Chen, P. Peng, C. Li, X. Chang, and Y. Wu, "Trace element transformations and partitioning during the roasting of pyrite ores in the sulfuric acid industry", J. Hazard. Mater., vol. 167, no. 1-3, pp. 835-845.
[http://dx.doi.org/10.1016/j.jhazmat.2009.01.067] [PMID: 19261379] ]. Therefore, the recycling of nonferrous metals in the cinders, especially gold and silver, is also an important research direction [12J. Wei, G. Yan, B. Guo, and G. Gao, "Extracting gold from pyrite roster cinder by ultra-fine-grinding and resin-in-pulp", J. Cent. South Univ. T., vol. 10, no. 1, pp. 27-31.
[http://dx.doi.org/10.1007/s11771-003-0065-z] -14J. Ding, J. Sun, P. Qian, Z. Pan, S. Ye, Q. Li, C. Wang, and Y. Chen, "Experimental study on recovering valuable metals from pyrite cinder by chloridizing roast", Comp, Applied, Chemistry, vol. 29, no. 3, pp. 255-261. [In Chinese].].
Pyrite usually contains a certain amount of arsenic, and most of the arsenic will get into the furnace gas in the process of roasting, then into the acid sludge, waste water and sulfuric acid through gas washing. However, there will still be a small amount of arsenic in the cinders [15W. Liu, "Sulfuric acid residue utilization whereabouts during the analysis of arsenic", Sichuan nonferrous metals, vol. 2, pp. 55-57. [In Chinese].]. The arsenic in the cinders is not only an important cause of environmental pollution, but also hinders the reuse of cinders as an important secondary resource [16T. Vamerali, M. Bandiera, and G. Mosca, "In situ phytoremediation of arsenic- and metal-polluted pyrite waste with field crops: Effects of soil management", Chemosphere, vol. 83, no. 9, pp. 1241-1248.
[http://dx.doi.org/10.1016/j.chemosphere.2011.03.013] [PMID: 21470658] ]. Arsenic into molten iron in the smelting process greatly decreases steel’s thermoplastic property and induces the steel crack easily in the stress processing [17J. Wang, L. Luo, H. Kong, and L. Zhou, "The arsenic removal from molten steel", High temp. mat. processes, vol. 30, pp. 171-173.]. Therefore, it should be removed before the reuse of cinders. The arsenic usually exists in the form of arsenate or arsenious oxide, and is mainly removed by acid leaching [18S. Bai, S. Wen, H. Zhao, and C. Lv, "Arsenic removal from pyrite cinders by oxidizing roasting with sodium hydroxide addition", Adv. Mat. Res., vol. 881-883, pp. 1655-1659., 19Y. Li, Z. Xu, and G. Zhang, "Study on the removal of arsenic in pyrite cinder by acid leaching", Adv. Mat. Res., vol. 781-784, pp. 2114-2119.]. There will be substantial amount of iron, copper and zinc into the leaching solution due to the poor selectivity of acid leaching, which will bring great difficulties to the following processing of solution treatment and increase the production cost. In view of the requirements of environmental protection and the simplification of the subsequent treatment process, this paper used the alkalic leaching method to selectively remove arsenic in the pyrite cinders, and arsenic in the leaching solution was treated harmlessly by ferric slat precipitation.
2. MATERIALS AND EXPERIMENTAL
2.1. Materials
The pyrite cinders used in this study were taken from one sulfuric acid plant in China. The main chemical compositions of the cinders were determined by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES, optima 8000) and Atomic Absorption Spectroscopy (AAS, WFX-130A), as the results are shown in Table (1). The sample was examined by X-ray diffraction (XRD, X’Pert PRO MPD) as shown in Fig. (1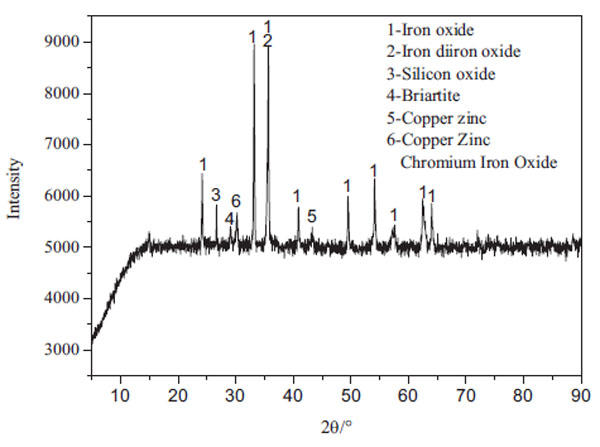 ). According to the analysis results, the main component of the cinders is Fe2O3, Fe3O4 and SiO2. The content of total ferric is 59.39%, in which hematite (Fe2O3) is 72.9% and magnetite (Fe3O4) is 17.3%. In addition, the zinc content is 1.51% and gold is 1.4g/t. Therefore, the pyrite cinder has a great recycling value. However, it is not conducive to reuse the cinders due to 0.45% arsenic content. The arsenic needs to be removed before recycling the cinder.
). According to the analysis results, the main component of the cinders is Fe2O3, Fe3O4 and SiO2. The content of total ferric is 59.39%, in which hematite (Fe2O3) is 72.9% and magnetite (Fe3O4) is 17.3%. In addition, the zinc content is 1.51% and gold is 1.4g/t. Therefore, the pyrite cinder has a great recycling value. However, it is not conducive to reuse the cinders due to 0.45% arsenic content. The arsenic needs to be removed before recycling the cinder.
 |
Fig. (1) XRD pattern of the pyrite cinders. |
2.2. Experiment
A certain amount of cinders with fine grinding and alkaline solution was put into a 250mL erlenmeyer flask, which was heated in a water bath pot. When the bath temperature went up to the specified temperature, the time was set as zero. After leaching and filtration, the residue was dried and determined by XRD, ICP-OES, AAS and SEM-EDS (JSM-7001F+INCA-MAX). The composition in the solution was analyzed by ICP-OES and AAS.
The leaching solution and washing solution were collected after each experiment. A specific amount of mixed liquid was taken in an Erlenmeyer flask and hydrogen peroxide was added. The solution pH was adjusted using sulfuric acid at room temperature (25 ○C). Then, the ferric sulfate was added and stirred to dissolve. After the precipitation, the pulp was filtrated. The content of arsenic in the filtrate was measured by ICP-OES, and the leaching residue was analyzed by XRD and SEM-EDS.
3. RESULTS AND DISCUSSION
The surface morphology of the pyrite cinders was observed by a scanning electron microscope, as shown in Fig. (2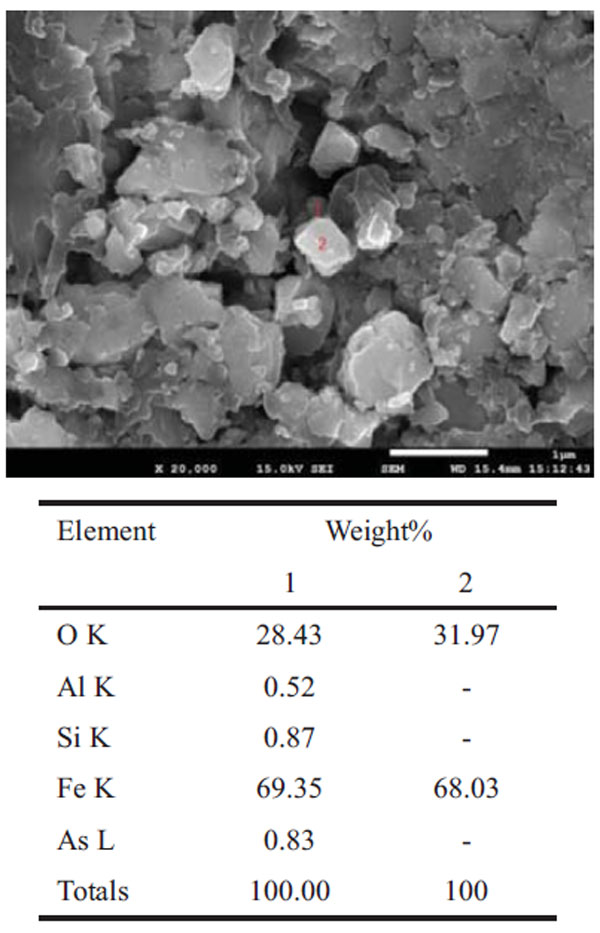 ). The two electronic results of area 1 show the content of arsenic to be 0.83%, which may exist in the form of ferric arsenate or arsenic oxide. The arsenic oxide can react with alkaline in the leaching solution. In addition, the zinc and lead can also be leached into the solution. However, the results of the analysis show that the solution almost contained no lead, and the lead content in the leaching residue changed slightly.
). The two electronic results of area 1 show the content of arsenic to be 0.83%, which may exist in the form of ferric arsenate or arsenic oxide. The arsenic oxide can react with alkaline in the leaching solution. In addition, the zinc and lead can also be leached into the solution. However, the results of the analysis show that the solution almost contained no lead, and the lead content in the leaching residue changed slightly.
 |
Fig. (2) SEM-EDS results of the pyrite cinders. |
3.1. The Arsenic Leaching from Pyrite Cinders
3.1.1. Effect of the Particle size on the Leaching of Arsenic
The pyrite cinders were finely ground to the size 0.074mm. The content of As and Zn in the leaching residue is shown in Fig. (3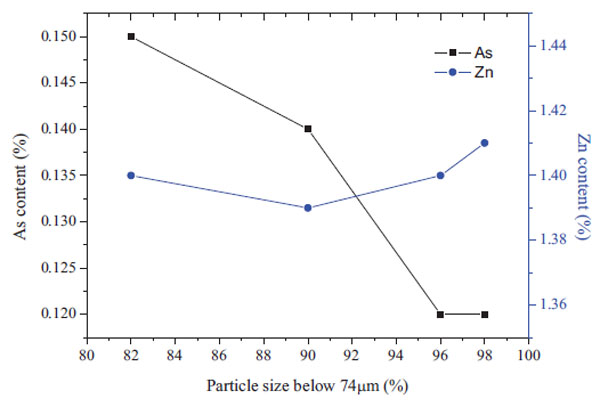 ) under the conditions of 80°C, S/L=1:5, 1mol/L NaOH, 3 hours of leaching time. Fig. (3
) under the conditions of 80°C, S/L=1:5, 1mol/L NaOH, 3 hours of leaching time. Fig. (3 ) indicates that the particle size influenced the leaching of As and Zn. The arsenic content in the residue decreased with the decrease in fine quality of the mineral. When the particles size was less than 96%, the arsenic decreased rapidly with the decreasing size. While the size under 0.074mm accounted for more than 96%, the As content changed slightly with further grinding. With regard to Zn content, the change in Zn was not obvious. This indicates that most of the zinc was exposed to leaching solution. Therefore, the size under 0.074mm accounted for 96% of the optimum fineness.
) indicates that the particle size influenced the leaching of As and Zn. The arsenic content in the residue decreased with the decrease in fine quality of the mineral. When the particles size was less than 96%, the arsenic decreased rapidly with the decreasing size. While the size under 0.074mm accounted for more than 96%, the As content changed slightly with further grinding. With regard to Zn content, the change in Zn was not obvious. This indicates that most of the zinc was exposed to leaching solution. Therefore, the size under 0.074mm accounted for 96% of the optimum fineness.
 |
Fig. (3) Effect of the particle size on the arsenic content in leaching residue.(80°C,3h,1mol/LNaOH,S/L=1:5) |
3.1.2. Effect of the Temperature on the Leaching of Arsenic
Temperature showed an significant influence on the leaching efficiency, so the effect of temperature between 25-90°C was studied under the conditions of S/L=1:5, 1mol/L NaOH, 3 hours of leaching time, as shown in Fig. (4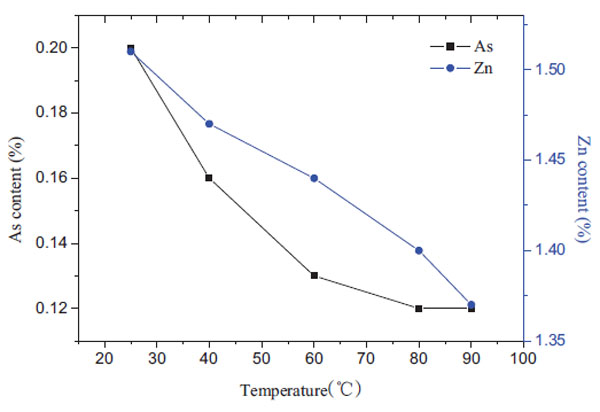 ). The results show that the content of arsenic in leaching residue decreased rapidly with the increase in temperature. Especially when the temperature was less than 80°C, the arsenic content changed from 0.25% at 25°C to 0.12% at 80°C. While it was more than 80°C, the arsenic content reduced slightly, which basically maintained at 0.12% at 90°C. In addition, the zinc content in the residue decreased with the increase in temperature at temperature more than 80°C. Therefore, it is beneficial to reduce the zinc loss at temperature below 80°C.
). The results show that the content of arsenic in leaching residue decreased rapidly with the increase in temperature. Especially when the temperature was less than 80°C, the arsenic content changed from 0.25% at 25°C to 0.12% at 80°C. While it was more than 80°C, the arsenic content reduced slightly, which basically maintained at 0.12% at 90°C. In addition, the zinc content in the residue decreased with the increase in temperature at temperature more than 80°C. Therefore, it is beneficial to reduce the zinc loss at temperature below 80°C.
 |
Fig. (4) Effect of temperature on the arsenic content in leaching residue. (3h 1mol/LNaOH S/L=1:5) |
3.1.3. Effect of the Alkaline Concentration on the Leaching of Arsenic
The concentration of NaOH solution is an important factor affecting the leaching behavior of arsenic in the cinders. Fig. (5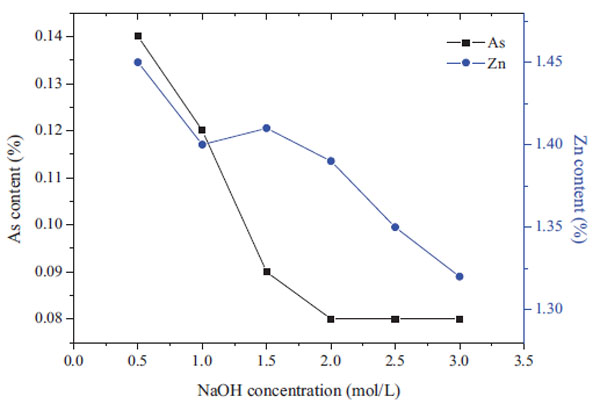 ) shows the changes in arsenic and zinc contents in the residue with the increase in the sodium hydroxide concentration. As observed, the arsenic content decreased gradually with the increase in the alkaline concentration. The arsenic content decreased to 0.08% when the NaOH concentration increased to 2.0mol/L. As the alkaline concentration further increased, the arsenic content changed indistinctly. However, the zinc content decreased gradually with the increase in the NaOH concentration, from 1.46% at 0.5mol/L to 1.3% at 3.0mol/L.
) shows the changes in arsenic and zinc contents in the residue with the increase in the sodium hydroxide concentration. As observed, the arsenic content decreased gradually with the increase in the alkaline concentration. The arsenic content decreased to 0.08% when the NaOH concentration increased to 2.0mol/L. As the alkaline concentration further increased, the arsenic content changed indistinctly. However, the zinc content decreased gradually with the increase in the NaOH concentration, from 1.46% at 0.5mol/L to 1.3% at 3.0mol/L.
 |
Fig. (5) Effect of alkaline concentration on the arsenic content in leaching residue. (3h,80°C,S/L=1:5) |
3.1.4. Effect of S/L on the Leaching of Arsenic
Fig. (6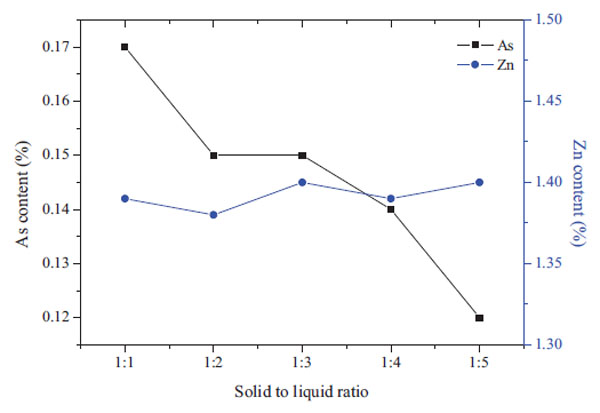 ) shows the effect of the solid-liquid ratio (S/L) on the arsenic and zinc contents in the leaching residue. The arsenic content decreased from 0.17% to 0.11% as the S/L increased from 1:1 to 1:5. However, the effect of S/L on zinc content was not obvious, therefore, the increase of the S/L ratio is beneficial to the arsenic leaching.
) shows the effect of the solid-liquid ratio (S/L) on the arsenic and zinc contents in the leaching residue. The arsenic content decreased from 0.17% to 0.11% as the S/L increased from 1:1 to 1:5. However, the effect of S/L on zinc content was not obvious, therefore, the increase of the S/L ratio is beneficial to the arsenic leaching.
 |
Fig. (6) Effect of S/L on the arsenic content in leaching residue. (3h, 80°C, 1mol/LNaOH) |
3.1.5. Effect of Reaction time on the Leaching of Arsenic
Fig. (7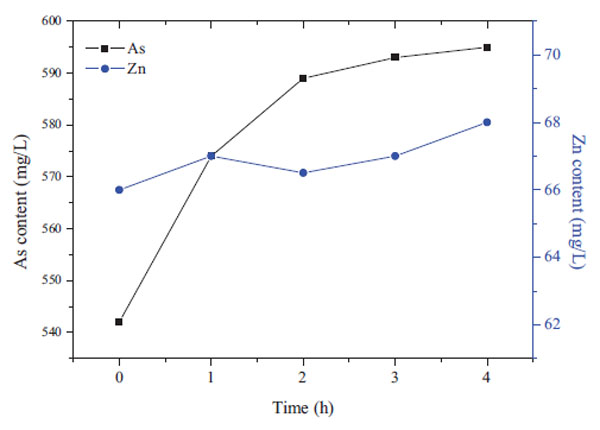 ) shows changes in the arsenic and zinc concentrations in the solution with time. At the beginning of the reaction, the leaching rate of arsenic increased rapidly, and the concentration reached to 570mg/L. As the reaction time extended to 4h, the arsenic concentration only increased to 595mg/L. However, the Zn concentration increased gradually with time, which even accelerated the trend after 2h. Therefore, the leaching time should be strictly controlled to reduce the zinc loss.
) shows changes in the arsenic and zinc concentrations in the solution with time. At the beginning of the reaction, the leaching rate of arsenic increased rapidly, and the concentration reached to 570mg/L. As the reaction time extended to 4h, the arsenic concentration only increased to 595mg/L. However, the Zn concentration increased gradually with time, which even accelerated the trend after 2h. Therefore, the leaching time should be strictly controlled to reduce the zinc loss.
 |
Fig. (7) Effect of leaching time on the arsenic concentration in leaching solution. (80°C, S:L=1:5, 1mol/LNaOH) |
In summary, alkaline concentration and temperature have an important effect on the leaching efficiency of arsenic. Most of the arsenic in pyrite cinders can be alkalic leached under the conditions of 80°C,with 96% fineness, 2mol/L NaOH, S:L=1:5 and 3h reaction time. The residues still contained about 0.08% arsenic after the verification test, which is possibly in the form of iron arsenate or being wrapped.
3.2. The Arsenic Precipitation in the Leaching Solution
The precipitation method is widely used for the removal of arsenic in solution, in which ferric salt precipitation is one of the most common techniques [20B. Van der Bruggen, and C. Vandecasteele, "Removal of pollutants from surface water and groundwater by nanofiltration: Overview of possible applications in the drinking water industry", Environ. Pollut., vol. 122, no. 3, pp. 435-445.
[http://dx.doi.org/10.1016/S0269-7491(02)00308-1] [PMID: 12547533] ]. Many researches show that several factors including arsenic concentration, pH and the molar ratio of iron to arsenic (Fe/As) affect the removal of arsenic [21S.R. Wickramasinghe, B.B. Han, Z. Zimbron, Z. Shen, and M.N. Karim, "Arsenic removal by coagulation and filtration: comparison of groundwaters from the United States and Bangladesh", Desalination, vol. 169, no. 3, pp. 231-244.
[http://dx.doi.org/10.1016/S0011-9164(04)00530-2] -23A. Zouboulis, and I. Katsoyiannis, "Removal of arsenates from contaminated water by coagulation-direct filtration", Sep. Sci. Technol., vol. 37, no. 12, pp. 2859-2873.
[http://dx.doi.org/10.1081/SS-120005470] ]. Fe(OH)2 and Fe(OH)3 are produced by the hydrolysis of ferric salt in water, which can not only react with arsenic to produce precipitation, but also adsorb the arsenic in the solution [24K. Müller, V.S. Ciminelli, M.S. Dantas, and S. Willscher, "A comparative study of As(III) and As(V) in aqueous solutions and adsorbed on iron oxy-hydroxides by Raman spectroscopy", Water Res., vol. 44, no. 19, pp. 5660-5672.
[http://dx.doi.org/10.1016/j.watres.2010.05.053] [PMID: 20599245] , 25A.C. Ladeira, and V.S. Ciminelli, "Adsorption and desorption of arsenic on an oxisol and its constituents", Water Res., vol. 38, no. 8, pp. 2087-2094.
[http://dx.doi.org/10.1016/j.watres.2004.02.002] [PMID: 15087189] ]. Because the toxicity and mobility of As (III) are much higher than that of As (V), the As (III) is usually oxidized to As (V) before arsenic precipitation [26W. Driehaus, "Oxidation of arsenate (III) with manganese oxides in water treatment", Water Res., vol. 29, pp. 297-305.
[http://dx.doi.org/10.1016/0043-1354(94)E0089-O] ]. In this study, the As (III) was firstly oxidized to As (V) using hydrogen peroxide at room temperature (25°C), then the arsenic was removed from the leaching solution by ferric sulfate.
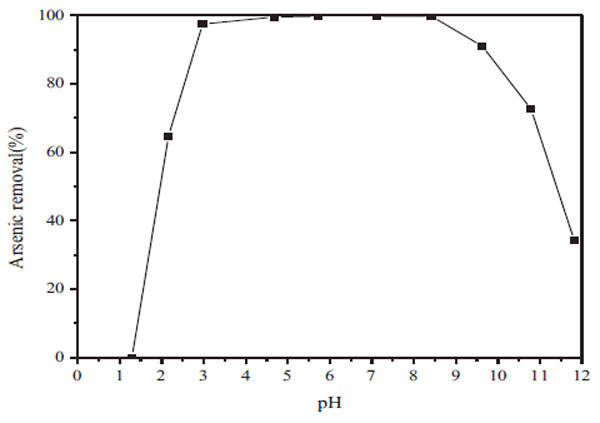 |
Fig. (8) Effect of pH on the arsenic removal efficiency (Fe/As=2). |
3.2.1. Effect of pH on the Arsenic Removal Efficiency
The effect of pH value within the range of 1-12 on the arsenic removal is shown in Fig. (8 ). The results show that no precipitation was produced with the pH below 1.5; the removal rate of arsenic was about 64% at pH=2.1. When the pH reached to 4.6, the arsenic removal was more than 99% and the arsenic concentration was observed to be less than 5mg/L. Because the ferric arsenate was found to equilibrate with two-line ferrihydrite as the pH>7 [27J.F. Leberre, R. Gauvin, and G.P. Demopoulos, "Characterization of poorly-crystalline ferric arsenate precipitated from equimolar Fe(III)-As(V) solutions in the pH range 2 to 8", Metall. Mater. Trans., B, Process Metall. Mater. Proc. Sci., vol. 38B, pp. 751-762.
). The results show that no precipitation was produced with the pH below 1.5; the removal rate of arsenic was about 64% at pH=2.1. When the pH reached to 4.6, the arsenic removal was more than 99% and the arsenic concentration was observed to be less than 5mg/L. Because the ferric arsenate was found to equilibrate with two-line ferrihydrite as the pH>7 [27J.F. Leberre, R. Gauvin, and G.P. Demopoulos, "Characterization of poorly-crystalline ferric arsenate precipitated from equimolar Fe(III)-As(V) solutions in the pH range 2 to 8", Metall. Mater. Trans., B, Process Metall. Mater. Proc. Sci., vol. 38B, pp. 751-762.
[http://dx.doi.org/10.1007/s11663-007-9081-y] ]:
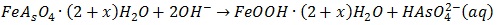 |
(1) |
The arsenic precipitation rate begins to decline when the pH is more than 8, which was only 91% at pH=9.6. Therefore, the pH plays an important role in arsenic precipitation from the leaching solution by ferric sulfate, and the optimal pH for arsenic removal is between the range of 4-8.
3.2.2. Effect of Fe/As on the Arsenic Removal Efficiency
Fig. (9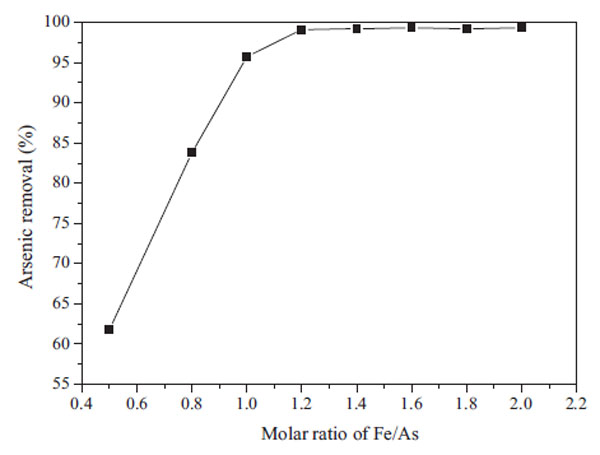 ) shows the removal rate of arsenic in solution with the increase of the Fe/As. The results indicate that the removal rate increased with the increase of the Fe/As. Only 95% arsenic was removed at Fe/As=1, which illustrates that the ferric sulfate did not equivalently react with the arsenic. When Fe/As reached to 1.2, the removal rate of arsenic in the leaching solution was over 99%.
) shows the removal rate of arsenic in solution with the increase of the Fe/As. The results indicate that the removal rate increased with the increase of the Fe/As. Only 95% arsenic was removed at Fe/As=1, which illustrates that the ferric sulfate did not equivalently react with the arsenic. When Fe/As reached to 1.2, the removal rate of arsenic in the leaching solution was over 99%.
 |
Fig. (9) Effect of Fe/As on the arsenic removal efficiency (pH=6-8). |
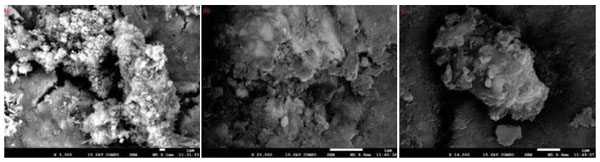 |
Fig. (10) SEM images of arsenic precipitation at pH=6-8,(a)Fe/As=0.8;(b)Fe/As=1;(c)Fe/As=2. |
More than 99% arsenic in the leaching solution can be removed by controlling the pH and Fe/As at room temperature. The surface morphological images of precipitations at Fe/As=0.8, 1, and 2 are shown in Fig. (10 ). It has been found that the precipitation mainly exists in the amorphous form agglomerated by superfine particles, whose stability is less than the crystalline ferric arsenate [28T. María, C.H. Pía, K.F. Elsa, R.G. Héctor, and A.G. Teófilo, "Equilibria of aqueous system phases containing arsenic + iron and arsenic + calcium at (323.15 and 343.15) K", J. Chem. Eng. Data, vol. 54, no. 11, pp. 3059-3068.
). It has been found that the precipitation mainly exists in the amorphous form agglomerated by superfine particles, whose stability is less than the crystalline ferric arsenate [28T. María, C.H. Pía, K.F. Elsa, R.G. Héctor, and A.G. Teófilo, "Equilibria of aqueous system phases containing arsenic + iron and arsenic + calcium at (323.15 and 343.15) K", J. Chem. Eng. Data, vol. 54, no. 11, pp. 3059-3068.
[http://dx.doi.org/10.1021/je900151s] , 29E. Krause, and V.A. Ettel, "Solubilities and stabilities of ferric arsenate compounds", Hydrometallurgy, vol. 22, no. 3, pp. 311-337.
[http://dx.doi.org/10.1016/0304-386X(89)90028-5] ]. The XRD result of arsenic precipitation proved this result as shown in Fig. (11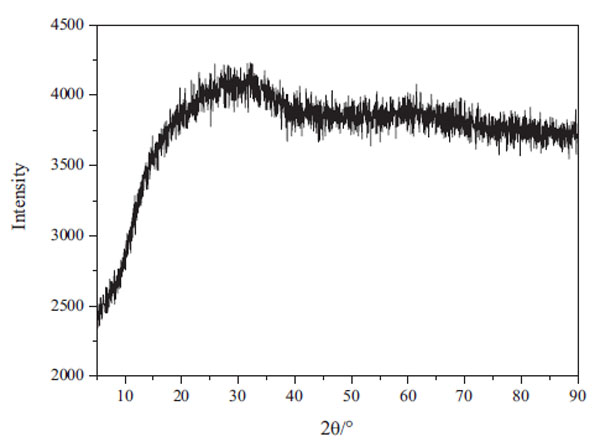 ). Min X. et al. pointed out that it can obtain ferric arsenate with higher crystallinity when the pH value was between 2-4, but the arsenic precipitation rate was only 84.6% at pH=4 [30X. Min, Y. Liao, L. Chai, Z. Yang, S. Xiong, L. Liu, and Q. Li, "Removal and stabilization of arsenic from anode slime by forming crystal scorodite", Trans. Nonferrous Met. Soc. China, vol. 25, pp. 1298-1306.
). Min X. et al. pointed out that it can obtain ferric arsenate with higher crystallinity when the pH value was between 2-4, but the arsenic precipitation rate was only 84.6% at pH=4 [30X. Min, Y. Liao, L. Chai, Z. Yang, S. Xiong, L. Liu, and Q. Li, "Removal and stabilization of arsenic from anode slime by forming crystal scorodite", Trans. Nonferrous Met. Soc. China, vol. 25, pp. 1298-1306.
[http://dx.doi.org/10.1016/S1003-6326(15)63728-1] ]. In this study, the removal rate at pH=3 was about 97%. Therefore, if the pH was less than 4, the arsenic in leaching was possibly not removed thoroughly, which also increased the acid consumption.
 |
Fig. (11) XRD result of arsenic precipitation. |
CONCLUSION
The alkalic leaching method was used to selectively remove the arsenic in the pyrite cinders, and the arsenic in the leaching solution was treated harmlessly by ferric slat precipitation. The arsenic in pyrite cinders can be removed through alkalic leaching under the conditions of 80°C, with 96% particle size, 2mol/L NaOH, S:L=1:5 and 3h of the reaction time, and the arsenic in the leaching residue was reduced to 0.08%. The arsenic in leaching solution was precipitated using ferric sulfate. More than 99% arsenic was removed at pH=4-8 and Fe/As>1.2, and the concentration in the solution reduced to less than 5mg/L. The precipitated arsenic may exist in the form of amorphous ferric arsenate under the precipitation conditions of pH=6-8 and Fe/As < 2.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We would like to thank the Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (Project No. 2012BAB08B04) and the National High Technology Research and Development Program of China (Project No. 2011AA06A104) for funding this project.
REFERENCES
| [1] | Z. Liu, and Y. Zheng, "Effect of Fe(II) on the formation of iron oxide synthesized from pyrite cinders by hydrothermal process", Powder Technol., vol. 209, pp. 119-123. [http://dx.doi.org/10.1016/j.powtec.2011.02.019] |
| [2] | A.V. Abdrakhimov, E.S. Abdrakhimova, and V.Z. Abdrakhimov, "Technical properties of roof tiles made of technogenic material with pyrite cinder", Glass Ceram., vol. 63, no. 3, pp. 130-132. [http://dx.doi.org/10.1007/s10717-006-0058-0] |
| [3] | I. Alp, H. Deveci, E.Y. Yazc, T. Türk, and Y. H. Süngün, "Süngün, Potential use of pyrite cinders as raw material in cement production: Results of industrial scale trial operations", J. Hazard. Mater., vol. 166, no. 1, pp. 144-149. |
| [4] | G. Fellet, L. Marchiol, D. Perosab, and G. Zerbi, "The application of phytoremediation technology in a soil contaminated by pyrite cinders", Ecol. Eng., vol. 31, pp. 207-214. [http://dx.doi.org/10.1016/j.ecoleng.2007.06.011] |
| [5] | Y. Zheng, and Z. Liu, "Preparation of monodispersed mica—teeous iron oxide pigment from pyrite cinders", Powder Technol., vol. 207, pp. 335-342. [http://dx.doi.org/10.1016/j.powtec.2010.11.015] |
| [6] | B. He, X. Tian, Y. Sun, C. Yang, Y. Zeng, Y. Wang, S. Zhang, and Z. Pi, "Recovery of iron oxide concentrate from high-sulfur and low-grade pyrite cinder using an innovative beneficiating process", Hydrometallurgy, vol. 104, pp. 241-246. [http://dx.doi.org/10.1016/j.hydromet.2010.06.009] |
| [7] | N. Tugrul, E.M. Derun, and M. Piskin, "Utilization of pyrite ash wastes by pelletization process", Powder Technol., vol. 176, no. 2, pp. 72-76. [http://dx.doi.org/10.1016/j.powtec.2007.01.012] |
| [8] | Z. Zou, A. Xuan, Z. Yan, Y. Wu, and N. Li, "Preparation of Fe304 particles from copper/iron Ore cinder and their microwave absorption properties", Chem. Eng. Sci., vol. 65, no. 1, pp. 160-164. [http://dx.doi.org/10.1016/j.ces.2009.06.003] |
| [9] | X. Xing, J. Zhang, Z. Wang, K. Jiao, X. Liu, and S. Ren, "Reduction of pyrite cinder pellets mixed with coal powder", J. Iron Steel Res. Int., vol. 21, no. 7, pp. 653-659. [http://dx.doi.org/10.1016/S1006-706X(14)60101-1] |
| [10] | Z. Lin, and Q. Ulf, "Predicting the mobility of Zn, Fe, Cu, Pb, Cd from roasted sulfide (pyrite) residues-a case study of wastes from the sulfuric acid industry in Sweden", Waste Manag., vol. 16, no. 8, pp. 671-681. [http://dx.doi.org/10.1016/S0956-053X(97)00009-3] |
| [11] | C. Yang, Y. Chen, P. Peng, C. Li, X. Chang, and Y. Wu, "Trace element transformations and partitioning during the roasting of pyrite ores in the sulfuric acid industry", J. Hazard. Mater., vol. 167, no. 1-3, pp. 835-845. [http://dx.doi.org/10.1016/j.jhazmat.2009.01.067] [PMID: 19261379] |
| [12] | J. Wei, G. Yan, B. Guo, and G. Gao, "Extracting gold from pyrite roster cinder by ultra-fine-grinding and resin-in-pulp", J. Cent. South Univ. T., vol. 10, no. 1, pp. 27-31. [http://dx.doi.org/10.1007/s11771-003-0065-z] |
| [13] | Y. Chang, X. Xu, and Y. Wang, "Arsenic removal and recovery of gold and silver by chloridizing roasting from high arsenic-bearing pyrite cinder", Nonferrous Metals, vol. 6, pp. 46-49. (In Chinese) |
| [14] | J. Ding, J. Sun, P. Qian, Z. Pan, S. Ye, Q. Li, C. Wang, and Y. Chen, "Experimental study on recovering valuable metals from pyrite cinder by chloridizing roast", Comp, Applied, Chemistry, vol. 29, no. 3, pp. 255-261. [In Chinese]. |
| [15] | W. Liu, "Sulfuric acid residue utilization whereabouts during the analysis of arsenic", Sichuan nonferrous metals, vol. 2, pp. 55-57. [In Chinese]. |
| [16] | T. Vamerali, M. Bandiera, and G. Mosca, "In situ phytoremediation of arsenic- and metal-polluted pyrite waste with field crops: Effects of soil management", Chemosphere, vol. 83, no. 9, pp. 1241-1248. [http://dx.doi.org/10.1016/j.chemosphere.2011.03.013] [PMID: 21470658] |
| [17] | J. Wang, L. Luo, H. Kong, and L. Zhou, "The arsenic removal from molten steel", High temp. mat. processes, vol. 30, pp. 171-173. |
| [18] | S. Bai, S. Wen, H. Zhao, and C. Lv, "Arsenic removal from pyrite cinders by oxidizing roasting with sodium hydroxide addition", Adv. Mat. Res., vol. 881-883, pp. 1655-1659. |
| [19] | Y. Li, Z. Xu, and G. Zhang, "Study on the removal of arsenic in pyrite cinder by acid leaching", Adv. Mat. Res., vol. 781-784, pp. 2114-2119. |
| [20] | B. Van der Bruggen, and C. Vandecasteele, "Removal of pollutants from surface water and groundwater by nanofiltration: Overview of possible applications in the drinking water industry", Environ. Pollut., vol. 122, no. 3, pp. 435-445. [http://dx.doi.org/10.1016/S0269-7491(02)00308-1] [PMID: 12547533] |
| [21] | S.R. Wickramasinghe, B.B. Han, Z. Zimbron, Z. Shen, and M.N. Karim, "Arsenic removal by coagulation and filtration: comparison of groundwaters from the United States and Bangladesh", Desalination, vol. 169, no. 3, pp. 231-244. [http://dx.doi.org/10.1016/S0011-9164(04)00530-2] |
| [22] | J.D. Chwirka, C. Colvin, J.D. Gomez, and P.A. Mueller, "Arsenic removal from drinking water using the coagulation/microfiltration process", J. Am. Water Works Assoc., vol. 96, no. 3, pp. 106-114. |
| [23] | A. Zouboulis, and I. Katsoyiannis, "Removal of arsenates from contaminated water by coagulation-direct filtration", Sep. Sci. Technol., vol. 37, no. 12, pp. 2859-2873. [http://dx.doi.org/10.1081/SS-120005470] |
| [24] | K. Müller, V.S. Ciminelli, M.S. Dantas, and S. Willscher, "A comparative study of As(III) and As(V) in aqueous solutions and adsorbed on iron oxy-hydroxides by Raman spectroscopy", Water Res., vol. 44, no. 19, pp. 5660-5672. [http://dx.doi.org/10.1016/j.watres.2010.05.053] [PMID: 20599245] |
| [25] | A.C. Ladeira, and V.S. Ciminelli, "Adsorption and desorption of arsenic on an oxisol and its constituents", Water Res., vol. 38, no. 8, pp. 2087-2094. [http://dx.doi.org/10.1016/j.watres.2004.02.002] [PMID: 15087189] |
| [26] | W. Driehaus, "Oxidation of arsenate (III) with manganese oxides in water treatment", Water Res., vol. 29, pp. 297-305. [http://dx.doi.org/10.1016/0043-1354(94)E0089-O] |
| [27] | J.F. Leberre, R. Gauvin, and G.P. Demopoulos, "Characterization of poorly-crystalline ferric arsenate precipitated from equimolar Fe(III)-As(V) solutions in the pH range 2 to 8", Metall. Mater. Trans., B, Process Metall. Mater. Proc. Sci., vol. 38B, pp. 751-762. [http://dx.doi.org/10.1007/s11663-007-9081-y] |
| [28] | T. María, C.H. Pía, K.F. Elsa, R.G. Héctor, and A.G. Teófilo, "Equilibria of aqueous system phases containing arsenic + iron and arsenic + calcium at (323.15 and 343.15) K", J. Chem. Eng. Data, vol. 54, no. 11, pp. 3059-3068. [http://dx.doi.org/10.1021/je900151s] |
| [29] | E. Krause, and V.A. Ettel, "Solubilities and stabilities of ferric arsenate compounds", Hydrometallurgy, vol. 22, no. 3, pp. 311-337. [http://dx.doi.org/10.1016/0304-386X(89)90028-5] |
| [30] | X. Min, Y. Liao, L. Chai, Z. Yang, S. Xiong, L. Liu, and Q. Li, "Removal and stabilization of arsenic from anode slime by forming crystal scorodite", Trans. Nonferrous Met. Soc. China, vol. 25, pp. 1298-1306. [http://dx.doi.org/10.1016/S1003-6326(15)63728-1] |




