- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Bioactive Compounds Journal
(Discontinued)
ISSN: 1874-8473 ― Volume 9, 2020
Methyl Ganoderic Acid DM: A Selective Potent Osteoclastogenesis Inhibitor
Jie Liu1, Jun Shiono1, Yukiko Tsuji2, Kuniyoshi Shimizu1, *, Ryuichiro Kondo1
Abstract
Increased osteoclastic bone resorption plays a central role in the pathogenesis of many bone diseases, and osteoclast inhibitors are the most widely used treatments for these diseases. Ganoderic acid DM, the main component of Ganoderma lucidum, has been known for its medicinal effects such as anti-androgen and anti-proliferative activities. In this study, we investigated the inhibitory effects of ganoderic acid DM and its analog (methyl ganoderic acid DM and 7-oxo-methyl ganoderic acid Z) on osteoclastogenesis using RAW264 cell in vitro. Methyl ganoderic acid DM blocked osteoclastogenesis completely at 12.5 μM with low cytotoxicity less than 30%. On the other hands, ganoderic acid DM blocked osteoclastogenesis completely at the higher concentration of 50 μM, but 7-oxo-methyl ganoderic acid Z did not up to 100 μM. These results implicated the carbonyl group at C-3 is essentially for selective osteoclastogenesis inhibitory activity, and methyl esters at C-26 should play an important role in enhancing its osteoclastogenesis inhibitory activity
Article Information
Identifiers and Pagination:
Year: 2009Volume: 2
First Page: 37
Last Page: 42
Publisher Id: TOBCJ-2-37
DOI: 10.2174/1874847300902010037
Article History:
Received Date: 10/7/2009Revision Received Date: 30/7/2009
Acceptance Date: 2/8/2009
Electronic publication date: 26/11/2009
Collection year: 2009
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Department of Forest and Forest Products Science, Faculty of Agriculture, Kyushu University, Fukuoka, 812-8581, Japan; Tel: +81-92-642-3002; Fax: +81-92-642-3002; E-mail: shimizu@agr.kyushu-u.ac.jp
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 10-7-2009 |
Original Manuscript | Methyl Ganoderic Acid DM: A Selective Potent Osteoclastogenesis Inhibitor | |
INTRODUCTION
The balance between bone resorption (by osteoclasts) and bone formation (by osteoblasts) maintains bone homeostasis in a process called bone remodeling [1Manolagas SC. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis Endocr Rev 2000; 21: 15-137.
[http://dx.doi.org/10.1210/er.21.2.115] [PMID: 10782361] ]. Bone is obviously resistant to dissolution, at least outside the body. Inside the body's highly active milieu, however, bone is remodeled at such a high speed that approximately 10% of the total bone content is replaced per year in adult humans [2Alliston T, Derynck R. Interfering with bone remodelling Nature 2002; 416: 686-7.
[http://dx.doi.org/10.1038/416686a] [PMID: 11961535] ]. Incremental changes in the rate of bone resorption can lead to bone disruption. This striking contrast emphasizes what an extraordinary and specific role osteoclasts play in the active maintenance of the bony skeleton. Large tartrate-resistant acid phosphatase (TRAP)-positive multinucleated cells (MNCs) that are hematopoietic in origin, osteoclasts are capable of resorbing bone [3Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation EndocrRev 992; 3: 66-80.
[http://dx.doi.org/10.1210/edrv-13-1-66] [PMID: 1555533] , 4Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation Bone 2007; 40: 251-64.
[http://dx.doi.org/10.1016/j.bone.2006.09.023] [PMID: 17098490] ].These multinucleated cells help dynamically remodel bones in coordination with osteoblasts, which deposit bone matrix. The excessive osteoclastic bone resorption relative to osteoblastic bone formation is often associated with osteopenic diseases including osteoporosis and rheumatoid arthritis. Osteoclastogenesis progresses through multiple stages, including differentiation, fusion, and activation (maturation) regulated by various factors, including cytokines, hormones, and other cells in the bone microenvironment. So the inhibition of osteoclast differentiation also has great clinical implications.
Osteoporosis is a very common disease accompanied by a high level of bone resorption, especially for postmenopausal women. Estrogen treatment, or hormone replacement therapy, is considered by many physicians to be the best method to prevent bone loss [5Anderson JJ, Garner SC. The molecular understanding of osteoclast differentiation Phytoestrogens and bone Baillieres Clin Endocrinol Metab 1998; 2: 543-57.
[http://dx.doi.org/10.1016/S0950-351X(98)80003-7] ]. However, many women do not tolerate the numerous side effects, or are concerned about the possible increased risk of uterine and/or breast cancer [6MacDonald BR, Gowen M. Emerging therapies in osteoporosis Best practice & research Clin Rheumatol 2001; 5: 483-96.
[http://dx.doi.org/10.1053/berh.2001.0162] [PMID: 11485342] -8Scheiber MD, Rebar RW. Isoflavones and postmenopausal bone health: a viable alternative to estrogen therapy Menopause 1999; 6: 233-41.
[http://dx.doi.org/10.1097/00042192-199906030-00010] [PMID: 10486794] ]. There thus remains a need for highly efficacious anti-resorptive agents with excellent safety and tolerability profiles. Several recent reports whose goal is to identify patterns for preventing osteoporosis through daily diet examined the effects of food components and their bioactive components on bone metabolism [9Mühlbauer RC, Li F. Nutrition Effect of vegetables on bone metabolism Nature 1999; 401: 343-4.
[http://dx.doi.org/10.1038/43824] [PMID: 10517630] -10Park CK, Lee Y, Chang EJ, et al. Bavachalcone inhibits osteoclast differentiation through suppression of NFATc1 induction by RANKL Biochem Pharmacol 2008; 75: 2175-82.].We also focused on identifying the lead compound for developmenting inhibitors of osteoclastogenesis among medicinal food stuffs.
The fungus G. lucidum (Reishi, Mannentake, or Lingzhi) has been used for centuries in East Asia to treat various human diseases such as hepatitis, hepatopathy, hypertension, nephritis, bronchitis, and cancers [11Yun TK. Update from Asia Asian studies on cancer chemoprevention Ann N Y Acad Sci 1999; 88: 57-192.
[http://dx.doi.org/10.1111/j.1749-6632.1999.tb08734.x] , 12Wasser SP, Weis AL. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: a modern perspective Crit Rev Immunol 1999; 9: 65-96.
[PMID: 9987601] ]. Its dried powder was especially popular as a cancer chemotherapy agent in the Imperial Court of ancient China [13Mizushina Y, Hanashima L, Yamaguchi T, et al. A mushroom fruiting body-inducing substance inhibits activities of replicative DNA polymerases Biochem Biophys Res Commun 998; 249: 7-22.]. G. lucidum has been reported to produce many bioactive oxygenated triterpenoids. Up to now, over 120 kinds of triterpenoids have been isolated from G. lucidum and the genus Ganoderma. Some of the triterpenoids such as ganoderic and lucidic acids, isolated from Ganoderma, have demonstrated cytotoxicity against mouse sarcoma and mouse lung carcinoma cells in vitro [14Min BS, Gao JJ, Nakamura N, Hattori M. Triterpenes from the spores of Ganoderma lucidum and their cytotoxicity against Meth-A and LLC tumor cells Chem Pharm Bull 2000; 48: 026-1033.
[http://dx.doi.org/10.1248/cpb.48.1026] [PMID: 10923835] ]. As part of our continuing search for biolo-gically active anti-osteoporotic compounds, we found that osteoclast differentiation was inhibited by the ethanol extracts of G. lucidum and ganoderic acid DM (1) which was isolated as one of the active compounds by bioassay-guided fractionation [15Miyamoto I, Liu J, Shimizu K, et al. Regulation of osteoclastogenesis by ganoderic acid DM isolated from Ganoderma lucidum Eur J Pharmacol 2009; 602: 7.
[http://dx.doi.org/10.1016/j.ejphar.2008.11.005] [PMID: 19026632] ]. Ganoderic acid DM (1) especially suppresses the expression of c-Fos and nuclear factor of activated T cells c1 (NFATc1). This suppression leads to the inhibition of dendritic cell-specific transmembrane protein (DC-STAMP) expression and reduces osteoclast fusion. These results prompted us to investigate the ability of ganoderic acid DM (1) analogs (2, 3) (Fig. 1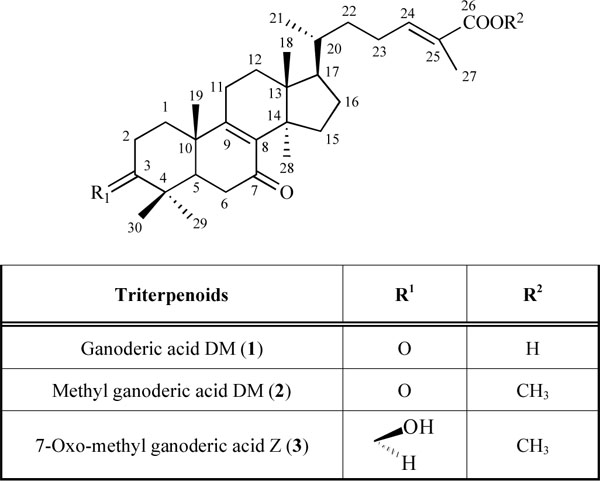 ) to inhibit osteoclast differentiation to determine what structural elements are important for the potent inhibition of osteoclast differentiation.
) to inhibit osteoclast differentiation to determine what structural elements are important for the potent inhibition of osteoclast differentiation.
 |
Fig. (1) Structures of triterpenoids (1-3). |
MATERIALS AND METHOD
Chemicals
The chemicals used were Dulbecco's modified Eagle's medium (DMEM) (Sigma, St. Louis, MO, USA), trimethylsilyldiazomethane (Tokyo chemical industry CO. LTD, Japan), activated charcoal, ethylenediaminetetraacetic acid (EDTA), dimethyl sulfoxide (DMSO), WST-1[4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate] (Wako, Osaka, Japan), glutamine (Nissui, Tokyo, Japan), penicillin, streptomycin, and trypsin (Invitrogen, Carlsbad, CA, USA). Fetal bovine serum (FBS), (-MEM was purchased from GIBCO BRL (Grand Island, NY, USA), soluble RANKL (sRANKL) was purchased from PeproTech EC Ltd (London, UK), and TNF-( was obtained from Roche Molecular Biochemical (Mannheim, Germany). Tartrate-resistent acid phosphatase (TRAP) staining kit was purchased from Sigma-Aldrich (St. Louis, MO, USA). Ganoderic acid DM (1)[16Liu J, Kurashiki K, Shimizu K, Kondo R. Structure-activity relationship for inhibition of 5a-reductase by triterpenoids isolated from Ganoderma lucidum Bioorg Med Chem 2006; 4: 8654-60.
[http://dx.doi.org/10.1016/j.bmc.2006.08.018] [PMID: 16962782] ]. was available from our previous works. The 13C NMR spectra of methyl ganoderic acid DM (2) and 7-oxo-methyl ganoderic acid Z (3)[17Li C, LiY M, Sun HH. New ganoderic acids, bioactive triterpenoid metabolites from the mushroom Ganoderma lucidum Nat Prod Res 2006; 20(11): 985-91.
[http://dx.doi.org/10.1080/14786410600921466] [PMID: 17050181] ] were measured. The molecular formula of these two compounds were estimated from its liquid chromatographs mass spectral-ion trap-time of flight (LCMS-IT-TOF) spectrum (2: [M+H+]at m/z 483.3469, calculated for C31H46O4, 482.3396; 3: [M+H+]at m/z 485.3504, calculated for C31H48O4, 484.3553).
Methyl ganoderic acid DM (2): ganoderic acid DM (10 mg) in methanol (1 ml) -benzene (3 ml) was added to trimethylsilyldiazomethane (1.5 ml) at room temperature. The mixture was stirred for 30 min at room temperature and concentrated to give the corresponding methyl esters of ganoderic acid DM as a white powder, then followed by preparative HPLC (column: Inertsil ODS-3(20 mm i.d. x 250 mm, GL Science, Inc. USA), methanol: water=80:20, flow rate: 10 ml/min) afforded methyl ganoderic acid DM (2) (Rt: 23 min). The molecular formula of methyl ganoderic acid DM (2) was determined to be C31H46O4 on the basis of the ion peak at m/z 483.3469 [M+H+] in LCMS-IT-TOF spectrum. The 1H NMR spectrum (Table1) of 2 indicated the presence of eight methyl singlets at δ 0.53, 0.78, 0.94, 0.96, 1.18, 1.68, 3.57, 6.60, an olefinic proton triplet at δ 6.60. The 13C NMR spectrum (Table 1) of 2 showed the presence of eight methyls (δ 5.86, 12.31, 17.87, 18.54, 21.36, 24.87, 25.32, 51.65), nine methylenes (δ 23.79, 25.58, 34.34, 28.54, 30.08, 31.83, 35.33, 34.82, 37.13), three methines (δ 36.17, 48.93, 50.36), four quaternary carbons (δ 39.38, 44.92, 47.2, 47.76), four olefinic carbons (δ 127.19, 139.48, 142.97, 162.67), two ketone carbons (δ 198.03, 214.63), and one carboxyl carbon (δ 168.71). Compare with the spectrum with 1, these 1H NMR and 13C NMR data (Table1) led us to suggest that compound 2 was the methyl esters of compound 1.
7-Oxo-methyl ganoderic acid Z (3): Conversion of C-3 keton of 2 to C-3 hydroxyl group was carried out by reduction with NaBH4. NaBH4 (10 mg) was added to 2 (10 mg) in methanol (3 ml) at room temperature. The mixture was stirred for 30 min at room temperature and the reaction was stopped by added acetic acid (1 ml). The mixture was concentrated in vacuo, then followed by preparative HPLC (column: Inertsil ODS-3, methanol: water=80:20, flow rate: 10 ml/min) afforded 7-oxo-methyl ganoderic acid Z (3) (Rt: 11 min) as a white powder. The molecular formula of 7-oxo-methyl ganoderic acid Z (3) was determined to be C31H48O4 on the basis of the ion peak at m/z 485.3504 [M+H+] in LCMS-IT-TOF spectrum. The 13C NMR spectrum (Table1) of 3 revealed total 31 carbon signals including 8 methyls, 9 methylenes, 4 methines, 4 quatemary carbons, 4 olefinic carbons, 1 carboxyl, 1 ketonecarbonyl, and 1 hydroxyl. Comparison of the 13C chemical shifts of 3 with those of 2 indicated that the structure of compound 3 was 3β-hydroxy-7-oxo-5α-lanosta-8, 24(E)-dien-26-oic acid.
Cell Cultures
RAW264 cells were maintained in DMEM with 10% FBS. All media were supplemented with 2 mM glutamine, 100 IU/ml penicillin, and 100 mg/ml streptomycin. Incubations were performed at 37ºC in 5% CO2 in humidified air. For osteoclast generation and other experiment, α-MEM medium was used.
TRAP-Positive Cell Staining
RAW264 cells were suspended in phenol α-MEM containing 10% FBS and plated at a concentration of 6.8(103 cells/well into a 96-well culture dish in the presence of 30 ng/ml RANKL and TNF-( (10 ng/ml), then incubated for 24 h [18Watanabe T, Kukita T, Kukita A, et al. Direct stimulation of osteoclastogenesis by MIP-1alpha: evidence obtained from studies using RAW264 cell clone highly responsive to RANKL J Endocrinol 2004; 80: 93-101.
[http://dx.doi.org/10.1677/joe.0.1800193] [PMID: 14709158] ]. Then, different concentrations of each compound were added to the cultures. After 3 days of culture, the cells were fixed and stained for TRAP using the TRAP staining kit according to the manufacturer’s instructions. TRAP staining cells with more than three nuclei were counted as osteoclast.
WST-1 Assay
RAW264 cells were suspended in phenol α-MEM containing 10% FBS and plated at a concentration of 6.8(103 cells/well into a 96-well culture dish in the presence of 30 ng/ml RANKL and TNF-( (10 ng/ml), then incubated for 24 h. Then, different concentrations of each compound were added to the cultures. After 3 days of culture, the number of viable cells was compared by WST-1WST-1[4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate] assay. Following incubation of cells for the above mentioned time, 10% WST-1 solution was added to each well and incubated at 37°C for three hours. Following incubation, plates were slightly shaken and immediately read at 450 nm with a scanning multiwell spectrophotometer.
Statistical Analysis
Data are reported as the mean ±S.D. Student’s t-test for cell experiments was done to determine any significant difference between the groups. Differences between means at the 1% confidence level (P<0.01) were considered to be statistically significant.
RESULTS
In our previous work, we found the extremely high potential of the ethanol extracts of G. lucidum as a regulator of bone resorption [15Miyamoto I, Liu J, Shimizu K, et al. Regulation of osteoclastogenesis by ganoderic acid DM isolated from Ganoderma lucidum Eur J Pharmacol 2009; 602: 7.
[http://dx.doi.org/10.1016/j.ejphar.2008.11.005] [PMID: 19026632] ]. Bioassay-guided fractionation led us to isolate ganoderic acid DM (1) as an inhibitor of osteoclastogenesis using RAW cells.
In general, dissociated form of carboxylic acids such as ganoderic acids is very unlikely to penetrate plasma membrane lipid bilayer. In neutral pH condition in medium, only small amount of the dissociated form of ganoderic acid DM (1) may enter into cytoplasm of RAW cells revealing the inhibition of osteoclastogenesis. If the specific lanostane-moiety in ganoderic acid DM (1) is essential for inhibition of osteoclastogenesis, but not carboxylic moiety at C-26, the change to the undissociated form of carboxylic moiety, more specifically, methyl ester-form (2) show more potent inhibitory activity than that of 1. Furthermore, if so, the structural modification of functional group in lanostane-moiety of 2 decreases its inhibitory activity.
Thus, we focused on ganoderic acid DM (1) and its structural related compounds (2, 3). To investigate whether these analog affect the differentiation of osteoclasts, we utilized the preosteoclastic cell line, RAW 264 cell, to study the effect of analog (2, 3) on the osteoclast-lineage. The cells were treated with each sample for 3 days. The results clearly indicated that not all compounds depressed the osteoclast differentiation (Figs. 2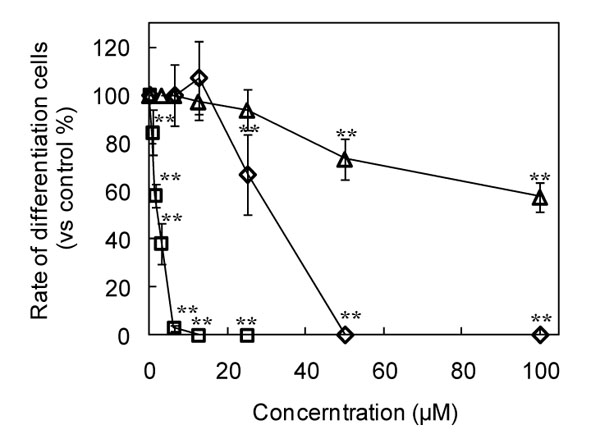 and 3
and 3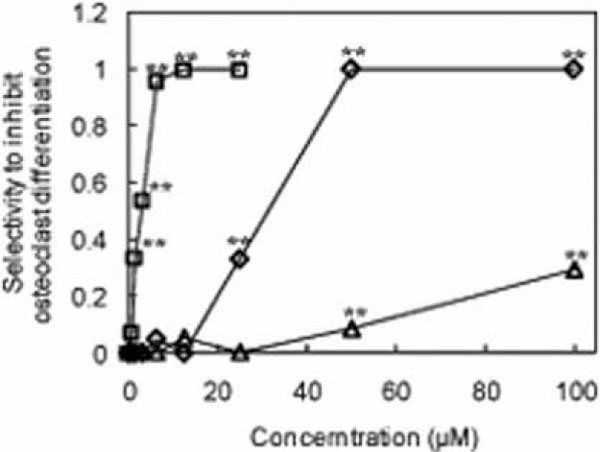 ). Interestingly, among these three compounds, methyl ganoderic acid DM (2) showed the strongest inhibitory activity to osteoclast differentiation than ganoderic acid DM (1) and 7-oxo-methyl ganoderic acid Z (3). Ganoderic acid DM (1) completely blocked the osteoclast differentiation at the concentration of 50 μM. 7-Oxo-methyl ganoderic acid Z (3) showed a low inhibitory activity on osteoclast differentiation even at the high concentration of 100 μM. Surprisingly, at the concentration of 12.5 μM, the osteoclast differentiation was completely blocked by methyl ganoderic acid DM (1). In contrast to methyl ganoderic acid DM (2), ganoderic acid DM (1) and 7-oxo-methyl ganoderic acid Z (3) showed no effect on osteoclast differentiation at this concentration. Almost complete inhibition of differentiation for ganoderic acid DM (1) was observed at 50 μM, but still remain 75% viable cells. The behavior of ganoderic acid DM (1) seemingly indicated that its inhibitory activity of differentiation not completely comes from its cytotoxicity. Like ganoderic acid DM (1), almost complete inhibition of differentiation for methyl ganoderic acid DM (2) was observed at 12.5 μM, but still remain more than 70% viable cells observed up to 20 μM. It should be noted that the limitation of solubility of methyl ganoderic acid DM (2) prevented future investigation more than 25 μM. Thus, we became aware that the inhibitory activity of methyl ganoderic acid DM (2) on differentiation was caused by not its cytotoxicity, but more like specific inhibition of differentiation. Furthermore, as speculated above, the easier penetration of 2 to inside of cells than 1 might enhance its inhibitory activity of osteoclastogenesis. This behavior, more specifically, selective inhibitory activity against differentiation, not viability, was important because safety is primarily consideration.
). Interestingly, among these three compounds, methyl ganoderic acid DM (2) showed the strongest inhibitory activity to osteoclast differentiation than ganoderic acid DM (1) and 7-oxo-methyl ganoderic acid Z (3). Ganoderic acid DM (1) completely blocked the osteoclast differentiation at the concentration of 50 μM. 7-Oxo-methyl ganoderic acid Z (3) showed a low inhibitory activity on osteoclast differentiation even at the high concentration of 100 μM. Surprisingly, at the concentration of 12.5 μM, the osteoclast differentiation was completely blocked by methyl ganoderic acid DM (1). In contrast to methyl ganoderic acid DM (2), ganoderic acid DM (1) and 7-oxo-methyl ganoderic acid Z (3) showed no effect on osteoclast differentiation at this concentration. Almost complete inhibition of differentiation for ganoderic acid DM (1) was observed at 50 μM, but still remain 75% viable cells. The behavior of ganoderic acid DM (1) seemingly indicated that its inhibitory activity of differentiation not completely comes from its cytotoxicity. Like ganoderic acid DM (1), almost complete inhibition of differentiation for methyl ganoderic acid DM (2) was observed at 12.5 μM, but still remain more than 70% viable cells observed up to 20 μM. It should be noted that the limitation of solubility of methyl ganoderic acid DM (2) prevented future investigation more than 25 μM. Thus, we became aware that the inhibitory activity of methyl ganoderic acid DM (2) on differentiation was caused by not its cytotoxicity, but more like specific inhibition of differentiation. Furthermore, as speculated above, the easier penetration of 2 to inside of cells than 1 might enhance its inhibitory activity of osteoclastogenesis. This behavior, more specifically, selective inhibitory activity against differentiation, not viability, was important because safety is primarily consideration.
Osteoclast differentiation, in response to receptor activator of NF-κB ligand (RANKL) and a tumor necrosis factor α (TNF-α), was inhibited by ganoderic acid DM (1) [15Miyamoto I, Liu J, Shimizu K, et al. Regulation of osteoclastogenesis by ganoderic acid DM isolated from Ganoderma lucidum Eur J Pharmacol 2009; 602: 7.
[http://dx.doi.org/10.1016/j.ejphar.2008.11.005] [PMID: 19026632] ]. The inhibitory activity of ganoderic acid DM is caused by especially suppressing the expression of c-Fos and nuclear factor of activated T cells c1 (NFATc1). This suppression leads to the inhibition of dendritic cell-specific transmembrane protein (DC-STAMP) expression and reduces osteoclast fusion. Obviously, the mechanistic investigation of osteoclast differentiation inhibitory activity by methyl ganoderic acid DM (1) (Fig. 2 ) needs to be determined for further application against osteoporosis-related disease.
) needs to be determined for further application against osteoporosis-related disease.
In Fig. (3 ), we defined selectivity to inhibit osteoclast differentiation by using the equality. selectivity to inhibit osteoclast differentiation = (the rate of cell viability (%) - the rate of differentiation cell (%) ) / the rate of cell viability (%).
), we defined selectivity to inhibit osteoclast differentiation by using the equality. selectivity to inhibit osteoclast differentiation = (the rate of cell viability (%) - the rate of differentiation cell (%) ) / the rate of cell viability (%).
When the selectivity to inhibit osteoclast differentiation equal 1, it means that no osteoclast differentiation cell can be found among the living cells. From Fig. (3 ), methyl ganoderic acid DM (2) showed the highest selectivity to inhibit osteoclast differentiation than ganoderic acid DM (1) and 7-oxo-methyl ganoderic acid Z (3). Methyl ganoderic acid DM (2) showed the value of 1 at 12.5 μM, ganoderic acid DM (1) showed the same value at 50 μM, and 7-oxo-methyl ganoderic acid Z (3) cannot give the same value even at 100 μM.
), methyl ganoderic acid DM (2) showed the highest selectivity to inhibit osteoclast differentiation than ganoderic acid DM (1) and 7-oxo-methyl ganoderic acid Z (3). Methyl ganoderic acid DM (2) showed the value of 1 at 12.5 μM, ganoderic acid DM (1) showed the same value at 50 μM, and 7-oxo-methyl ganoderic acid Z (3) cannot give the same value even at 100 μM.
DISCUSSION
Methyl ganoderic acid DM (2) and ganoderic acid DM (1), which showed higher selectivity to inhibit osteoclast differentiation, have a carbonyl group at C-3. In contrast to them, 7-oxo-methyl ganoderic acid Z, which showed low selectivity to inhibit osteoclast differentiation, has a hydroxyl group at C-3. These results suggested that a carbonyl group of the C-3 is essential to elicit the selectivity to inhibit osteoclast differentiation, and a methyl group at C-26 is needed to lower the cytotoxicity to osteoclast among these three compounds.
Osteoclasts appear uniquely adapted to produce the microenvironment and the biochemical milieu that are needed to resorb bone. Most notable of these is the cell membrane–associated protein termed receptor activator of RANKL, which is a member of the TNF family of cytokines. RANKL can then bind to its cognate receptor (RANK) on osteoclast precursors and, enhance the differentiation and fusion of these cells to produce functioning multinucleated osteoclasts. Concomitantly, eliminating the forming of osteoclasts means the bone resorption could be repressed. In this study, we found that methyl ganoderic acid DM (2) showed highly selective inhibition of osteoclastogenesis in RAW cells. Thus makes this compound can be used in osteoclastogenesis therapeutics.
It is not clear if the ingested methyl ganoderic acid DM (2) is absorbed into the biological system through the intestinal tract and delivered to the place where they are needed. The colonic microfloras convert most of these ingested triterpenoids into metabolites that then reach the circulation. Further biological evaluation of these triterpenoids is needed from only one aspect, but from a whole and dynamic perspective.
If the inhibitory activity of osteoclastic differentiation by the methyl ganoderic acid DM (2) from G. lucidum is also producible in human osteoclastic cells, it would be useful in a different sense since osteoclast play a crucial role in the osteopenic diseases including osteoporosis and rheumatoid arthritis.
Altogether our results illustrate the ability of some natural product-derived substances, ganoderic acid DM (1) and its analog (2) to inhibit the osteoclasts differentiation. For this kind of triterpenoids, a carbonyl group of the C-3 is essential to elicit the inhibition of osteoclast differentiation, and a methyl group at C-26 is needed to lower the cytotoxicity to osteoclast. Methyl ganoderic acid DM (2) should be a potential candidate for treating osteopenic diseases, such as osteoporosis and rheumatoid arthritis. Accumulation of the knowledge of the regulatory control of osteoclastogenesis on a molecular basis may provide a more rational and scientific approach to design safe and effective osteoclastogenesis control agents.
REFERENCES
| [1] | Manolagas SC. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis Endocr Rev 2000; 21: 15-137. [http://dx.doi.org/10.1210/er.21.2.115] [PMID: 10782361] |
| [2] | Alliston T, Derynck R. Interfering with bone remodelling Nature 2002; 416: 686-7. [http://dx.doi.org/10.1038/416686a] [PMID: 11961535] |
| [3] | Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation EndocrRev 992; 3: 66-80. [http://dx.doi.org/10.1210/edrv-13-1-66] [PMID: 1555533] |
| [4] | Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation Bone 2007; 40: 251-64. [http://dx.doi.org/10.1016/j.bone.2006.09.023] [PMID: 17098490] |
| [5] | Anderson JJ, Garner SC. The molecular understanding of osteoclast differentiation Phytoestrogens and bone Baillieres Clin Endocrinol Metab 1998; 2: 543-57. [http://dx.doi.org/10.1016/S0950-351X(98)80003-7] |
| [6] | MacDonald BR, Gowen M. Emerging therapies in osteoporosis Best practice & research Clin Rheumatol 2001; 5: 483-96. [http://dx.doi.org/10.1053/berh.2001.0162] [PMID: 11485342] |
| [7] | Santell RC, Chang YC, Nair MG, Helferich WG. Dietary genistein exerts estrogenic effects upon the uterus mammary gland and the hypothalamic/pituitary axis in rats J Nutr 997; 27: 263-9. [PMID: 9039826] |
| [8] | Scheiber MD, Rebar RW. Isoflavones and postmenopausal bone health: a viable alternative to estrogen therapy Menopause 1999; 6: 233-41. [http://dx.doi.org/10.1097/00042192-199906030-00010] [PMID: 10486794] |
| [9] | Mühlbauer RC, Li F. Nutrition Effect of vegetables on bone metabolism Nature 1999; 401: 343-4. [http://dx.doi.org/10.1038/43824] [PMID: 10517630] |
| [10] | Park CK, Lee Y, Chang EJ, et al. Bavachalcone inhibits osteoclast differentiation through suppression of NFATc1 induction by RANKL Biochem Pharmacol 2008; 75: 2175-82. |
| [11] | Yun TK. Update from Asia Asian studies on cancer chemoprevention Ann N Y Acad Sci 1999; 88: 57-192. [http://dx.doi.org/10.1111/j.1749-6632.1999.tb08734.x] |
| [12] | Wasser SP, Weis AL. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: a modern perspective Crit Rev Immunol 1999; 9: 65-96. [PMID: 9987601] |
| [13] | Mizushina Y, Hanashima L, Yamaguchi T, et al. A mushroom fruiting body-inducing substance inhibits activities of replicative DNA polymerases Biochem Biophys Res Commun 998; 249: 7-22. |
| [14] | Min BS, Gao JJ, Nakamura N, Hattori M. Triterpenes from the spores of Ganoderma lucidum and their cytotoxicity against Meth-A and LLC tumor cells Chem Pharm Bull 2000; 48: 026-1033. [http://dx.doi.org/10.1248/cpb.48.1026] [PMID: 10923835] |
| [15] | Miyamoto I, Liu J, Shimizu K, et al. Regulation of osteoclastogenesis by ganoderic acid DM isolated from Ganoderma lucidum Eur J Pharmacol 2009; 602: 7. [http://dx.doi.org/10.1016/j.ejphar.2008.11.005] [PMID: 19026632] |
| [16] | Liu J, Kurashiki K, Shimizu K, Kondo R. Structure-activity relationship for inhibition of 5a-reductase by triterpenoids isolated from Ganoderma lucidum Bioorg Med Chem 2006; 4: 8654-60. [http://dx.doi.org/10.1016/j.bmc.2006.08.018] [PMID: 16962782] |
| [17] | Li C, LiY M, Sun HH. New ganoderic acids, bioactive triterpenoid metabolites from the mushroom Ganoderma lucidum Nat Prod Res 2006; 20(11): 985-91. [http://dx.doi.org/10.1080/14786410600921466] [PMID: 17050181] |
| [18] | Watanabe T, Kukita T, Kukita A, et al. Direct stimulation of osteoclastogenesis by MIP-1alpha: evidence obtained from studies using RAW264 cell clone highly responsive to RANKL J Endocrinol 2004; 80: 93-101. [http://dx.doi.org/10.1677/joe.0.1800193] [PMID: 14709158] |